Levetiracetam composition, preparation method and application
A composition and preparation technology, applied in the field of levetiracetam composition and preparation, can solve problems such as poor patient compliance, and achieve the effects of convenient medication, good local tolerance, and reduced medication difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
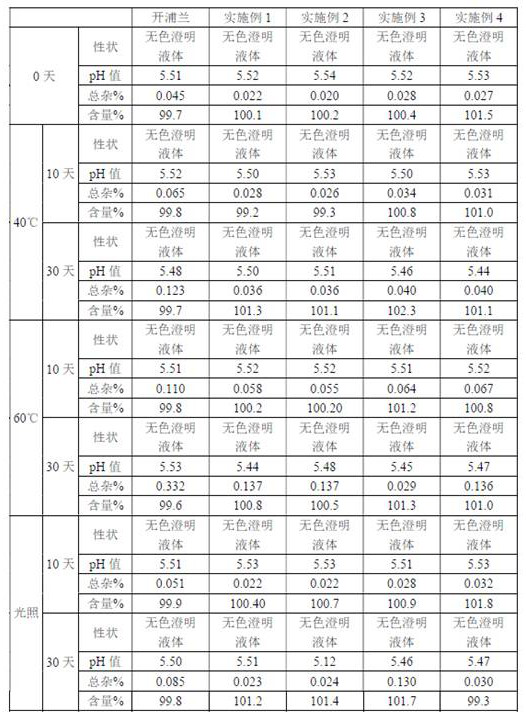
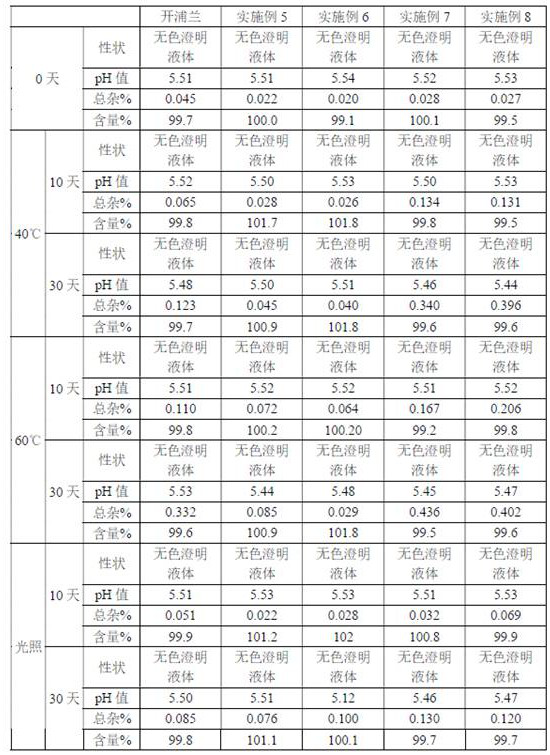
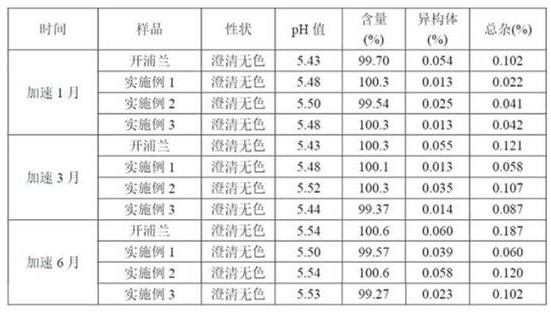
Examples
Embodiment 1
[0071] Present embodiment provides a kind of levetiracetam composition, and prescription is composed as follows:
[0072] Levetiracetam 500g Sodium acetate trihydrate 4g Sodium chloride 4g glacial acetic acid Appropriate amount Water for Injection 5000ml
[0073] The preparation process of the above-mentioned composition is as follows:
[0074] 1) Prepare 10% glacial acetic acid solution for later use;
[0075] 2) Take 3500ml of water for injection, put it in a container, keep stirring, add the prescribed amount of levetiracetam, sodium acetate trihydrate, sodium chloride in sequence, and keep stirring until completely dissolved;
[0076] 3) Use 10% glacial acetic acid to adjust the pH of the liquid to 5.5±0.3, make up to 5000ml with water for injection, and keep stirring;
[0077] 4) Filtration, fine filtration, potting in a 5ml glass bottle;
[0078] 5) 121°C, 15min damp heat sterilization;
[0079] 6) After the light inspection, l...
Embodiment 2
[0081] Present embodiment provides a kind of levetiracetam composition, and prescription is composed as follows:
[0082] Levetiracetam 250g Sodium acetate trihydrate 2g Sodium chloride 0.5g glacial acetic acid Appropriate amount Water for Injection 5000ml
[0083] The preparation process of the above-mentioned composition is as follows:
[0084] 1) Prepare 10% glacial acetic acid solution for later use;
[0085] 2) Take 3500ml of water for injection, put it in a container, keep stirring, add the prescribed amount of levetiracetam, sodium acetate trihydrate, sodium chloride in sequence, and keep stirring until completely dissolved;
[0086] 3) Use 10% glacial acetic acid to adjust the pH of the liquid to 5.5±0.3, make up to 5000ml with water for injection, and keep stirring;
[0087] 4) Filtration, fine filtration, potting in a 5ml glass bottle;
[0088] 5) 121°C, 15min damp heat sterilization;
[0089] 6) After the light inspection,...
Embodiment 3
[0091] Present embodiment provides a kind of levetiracetam composition, and prescription is composed as follows:
[0092] Levetiracetam 500g Sodium acetate trihydrate 4g Sodium chloride 12g glacial acetic acid Appropriate amount Water for Injection 5000ml
[0093] The preparation process of the above-mentioned composition is as follows:
[0094] 1) Prepare 10% glacial acetic acid solution for later use;
[0095] 2) Take 3500ml of water for injection, put it in a container, keep stirring, add the prescribed amount of levetiracetam, sodium acetate trihydrate, sodium chloride in sequence, and keep stirring until completely dissolved;
[0096] 3) Use 10% glacial acetic acid to adjust the pH of the liquid to 5.5±0.3, make up to 5000ml with water for injection, and keep stirring;
[0097] 4) Filtration, fine filtration, potting in a 5ml glass bottle;
[0098] 5) 121°C, 15min damp heat sterilization;
[0099] 6) After the light inspection, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com