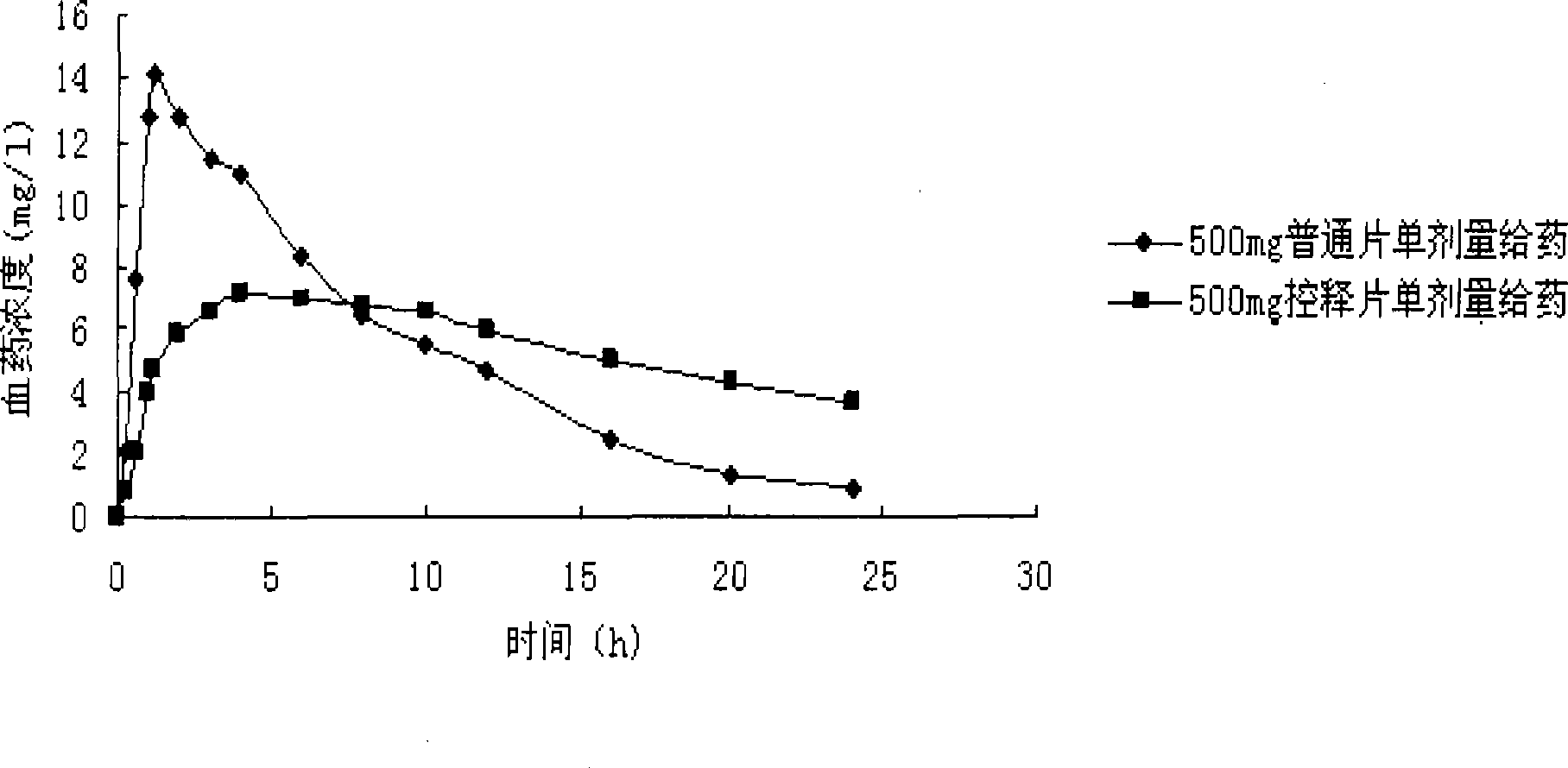
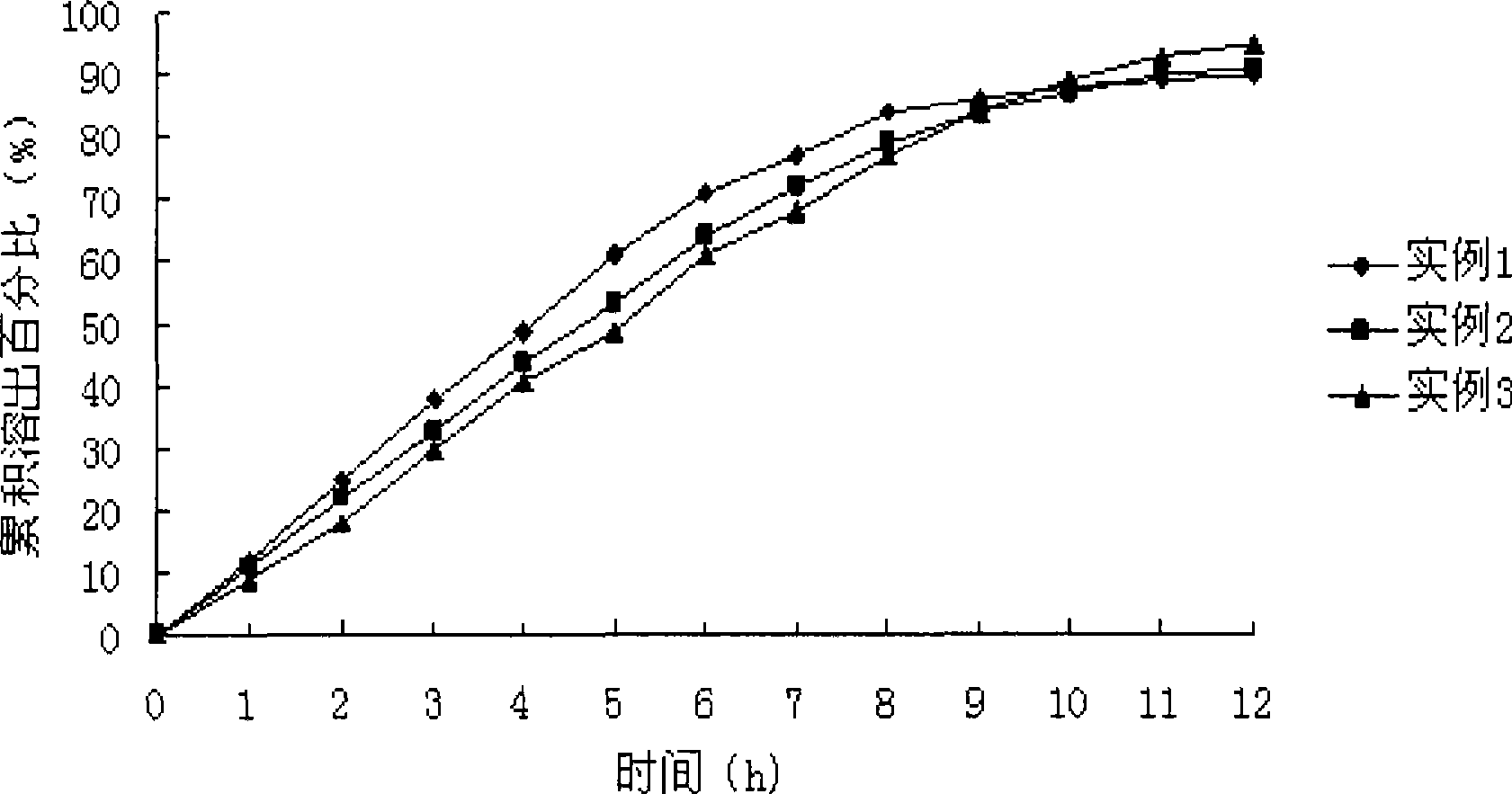
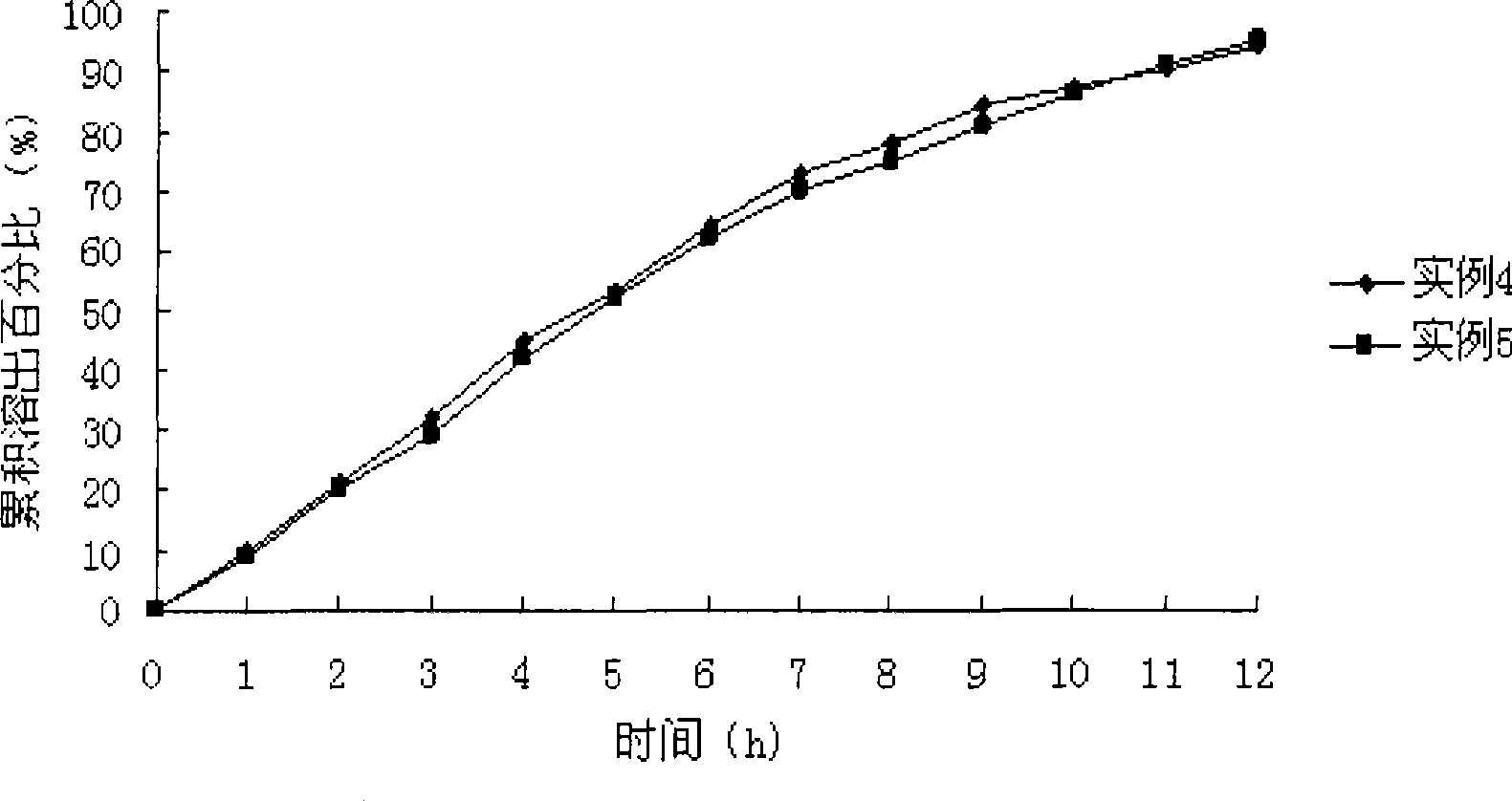
Levetiracetam osmotic pump controlled release tablet and preparation method thereof
An osmotic pump controlled release, tablet core technology, applied in the field of medicine, can solve the problem of no obvious dose correlation, and achieve the effect of continuous drug release, stable drug release, and elimination of peak-to-valley phenomenon.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Tablet prescription:
[0040] Levetiracetam 60%
[0041] Microcrystalline Cellulose 36.5%
[0042] PVP K30 2.5%
[0043] Magnesium Stearate 1.0%
[0044] ethanol, water
[0045] Semipermeable Membrane Prescription:
[0046] Cellulose acetate 80%
[0047] PEG-4000 20%
[0048] Preparation method: mix the prescribed amount of levetiracetam and microcrystalline cellulose according to the above ratio, mix PVP K30 and 30% ethanol to prepare a binder with a solid content of 10%, then wet granulate, and dry , sieved, added magnesium stearate and compressed into tablets to make tablet cores. Dissolve cellulose acetate and PEG-4000 in a mixed solution of acetone-water (mass ratio 95:5), release tablet cores in a coating pan for coating, and place the coated tablets in a dry box after coating Dry to cure the coating film. Then a suitable drug release hole is prepared on one side of the coating film by laser or mechanical method. Finally, the coated tablet is further coa...
Embodiment 2
[0050] Tablet prescription:
[0051] Levetiracetam 70%
[0052] Polyoxyethylene (molecular weight: 200,000) 21.5%
[0053] Lactose 6.5
[0054] Sodium Carboxymethyl Cellulose 1.0%
[0055] Magnesium Stearate 1.0%
[0056] ethanol, water
[0057] Semipermeable Membrane Prescription:
[0058] Cellulose acetate 75%
[0059] PEG-1500 25%
[0060] Preparation method: mix the prescribed amount of levetiracetam with polyoxyethylene (molecular weight: 200,000) and lactose according to the above ratio, and mix sodium carboxymethylcellulose with 30% ethanol to prepare a compound with a solid content of 2%. Binder, then wet granulation, drying, sieving, adding magnesium stearate and compressing into tablets to make tablet cores. Dissolve cellulose acetate and PEG-1500 in a mixed solution of acetone-water (mass ratio: 95:5), place the tablet core in a coating pan for coating, and place the coated tablet in a dry box after coating Dry to cure the coating film. Then a suitable dru...
Embodiment 3
[0062] Tablet prescription:
[0063] Levetiracetam 69%
[0064] Hypromellose 27.5%
[0065] PVP K30 2.5%
[0066] Magnesium Stearate 1.0%
[0067] ethanol, water
[0068] Semipermeable Membrane Prescription:
[0069] Cellulose acetate 75%
[0070] PEG-1500 25%
[0071] Preparation method: mix the prescribed amount of levetiracetam and hypromellose according to the above ratio, mix PVP K30 and 30% ethanol to prepare a binder with a solid content of 10%, and then wet granulate, Dry, sieve, add magnesium stearate and compress into tablets to make tablet cores. Dissolve cellulose acetate and PEG-1500 in a mixed solution of acetone-water (mass ratio: 95:5), place the tablet core in a coating pan for coating, and place the coated tablet in a dry box after coating Dry to cure the coating film. Then a suitable drug release hole is prepared on one side of the coating film by laser or mechanical method. Finally, the coated tablet is further coated with a non-functional coating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com