A kind of preparation method of levetiracetam key intermediate (s)-2-aminobutanamide salt
A technology of aminobutyramide salts and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of poor product yield and purity, difficulties in the preparation of by-products, and many impurities, and achieve easy control of reactions, simple recovery, and process safety high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
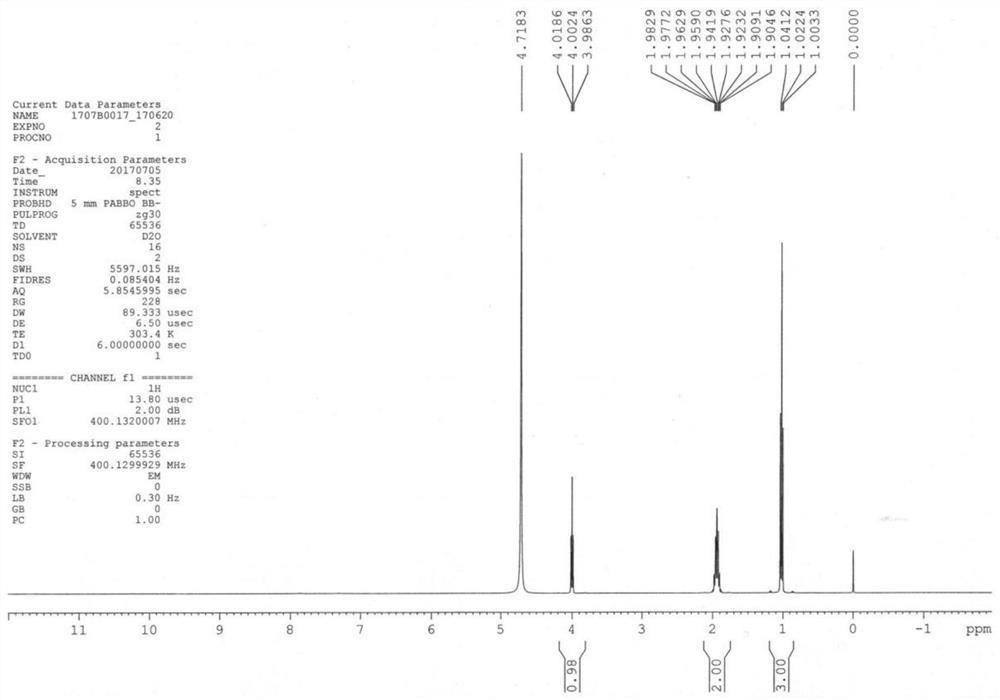
[0035] Add compound 1 (11.7g, 0.1mol) into 100mL of methanol, cool down to 0-5°C, pass ammonia gas to saturation, react at room temperature for 36h, after the reaction is complete, concentrate to dryness, add 50mL of methanol, and pass through ammonia gas for 1h , react at room temperature for 2 h, filter, concentrate to dryness, add 100 mL of methanol, dropwise add methanolic hydrochloric acid solution, dropwise, grow crystals for 1 h, filter, wash with methanol, and dry in vacuo to obtain compound 2 (12.5 g, molar yield 90.2%) ESI- MS(m / z)=103.2[M+H] + ; 1 HNMR (D 2 (2, 400MHz) δ: 1.0 (t, 3H), 1.9 (m, 2H), 4.2 (t, 1H), HPLC purity 99.9%, the proton nuclear magnetic resonance spectrum of compound 2 is as figure 1 shown.
Embodiment 2
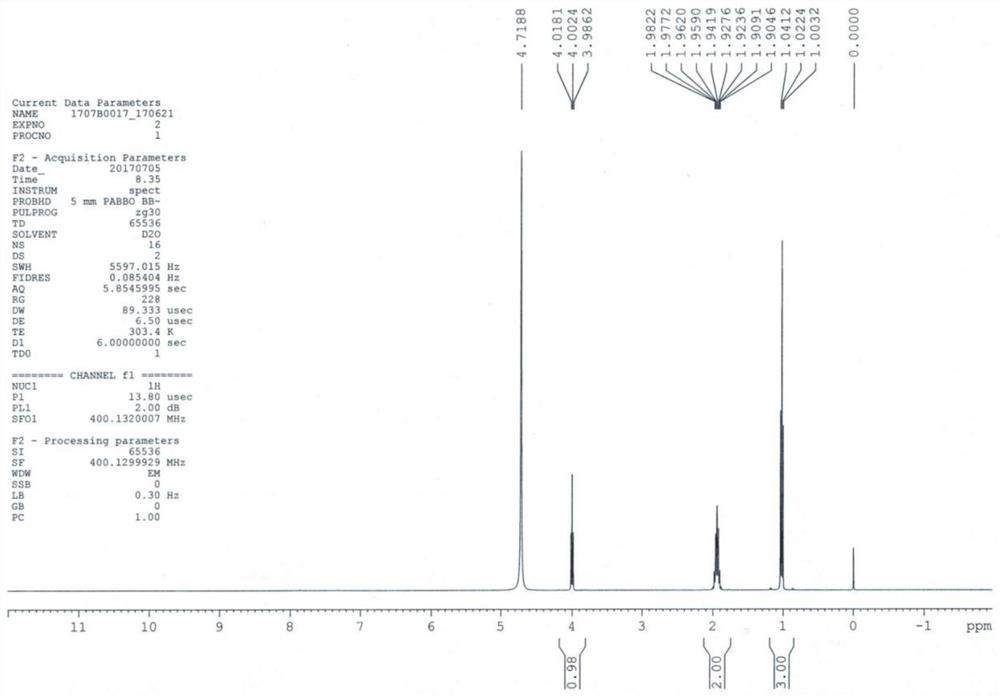
[0037] Add compound 1 (11.7g, 0.1mol) into 100mL of ethanol, cool down to 0-5°C, pass ammonia gas to saturation, react at room temperature for 36h, after the reaction is completed, concentrate to dryness, add 50mL of ethanol, and pass through ammonia gas for 1h , react at room temperature for 2 h, filter, concentrate to dryness, add 100 mL of ethanol, dropwise add ethanol hydrochloric acid solution, dropwise, grow crystals for 1 h, filter, wash with ethanol, and dry in vacuo to obtain compound 2 (12.7 g, molar yield 91.6%) ESI- MS(m / z)=103.2[M+H] + ; 1 HNMR (D 2 (2, 400MHz) δ: 1.0 (t, 3H), 1.9 (m, 2H), 4.2 (t, 1H), the HPLC purity is 99.9%, and the proton nuclear magnetic resonance spectrum of compound 2 is as figure 2 shown.
Embodiment 3
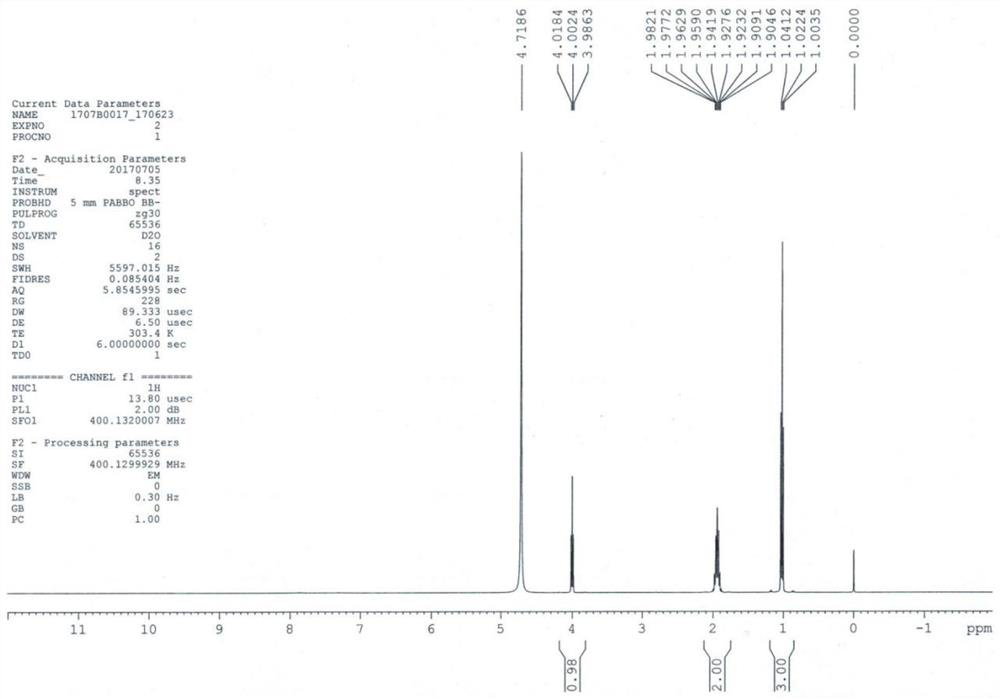
[0039] Add compound 1 (11.7g, 0.1mol) into 100mL of isopropanol, cool down to 0-5°C, pass ammonia gas to saturation, react at room temperature for 36h, after the reaction is complete, concentrate to dryness, add 50mL of isopropanol, pass through Add ammonia gas for 1 h, react at room temperature for 2 h, filter, concentrate to dryness, add 100 mL of isopropanol, add isopropanol hydrochloride solution dropwise, dropwise, grow crystals for 1 h, filter, wash with isopropanol, and dry in vacuo to obtain compound 2 (12.9 g, molar yield 92.8%) ESI-MS (m / z) = 103.2 [M+H] + ; 1 HNMR (D 2 (2, 400MHz) δ: 1.0 (t, 3H), 1.9 (m, 2H), 4.2 (t, 1H), the HPLC purity is 99.9%, and the proton nuclear magnetic resonance spectrum of compound 2 is as image 3 shown.
[0040] By embodiment 1~3 and Figure 1 ~ Figure 3 It can be seen that the preparation method of the levetiracetam key intermediate (S)-2-aminobutyramide salt of the present invention is simple to operate, and the yield and purity a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com