Patents
Literature
314results about How to "High response rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
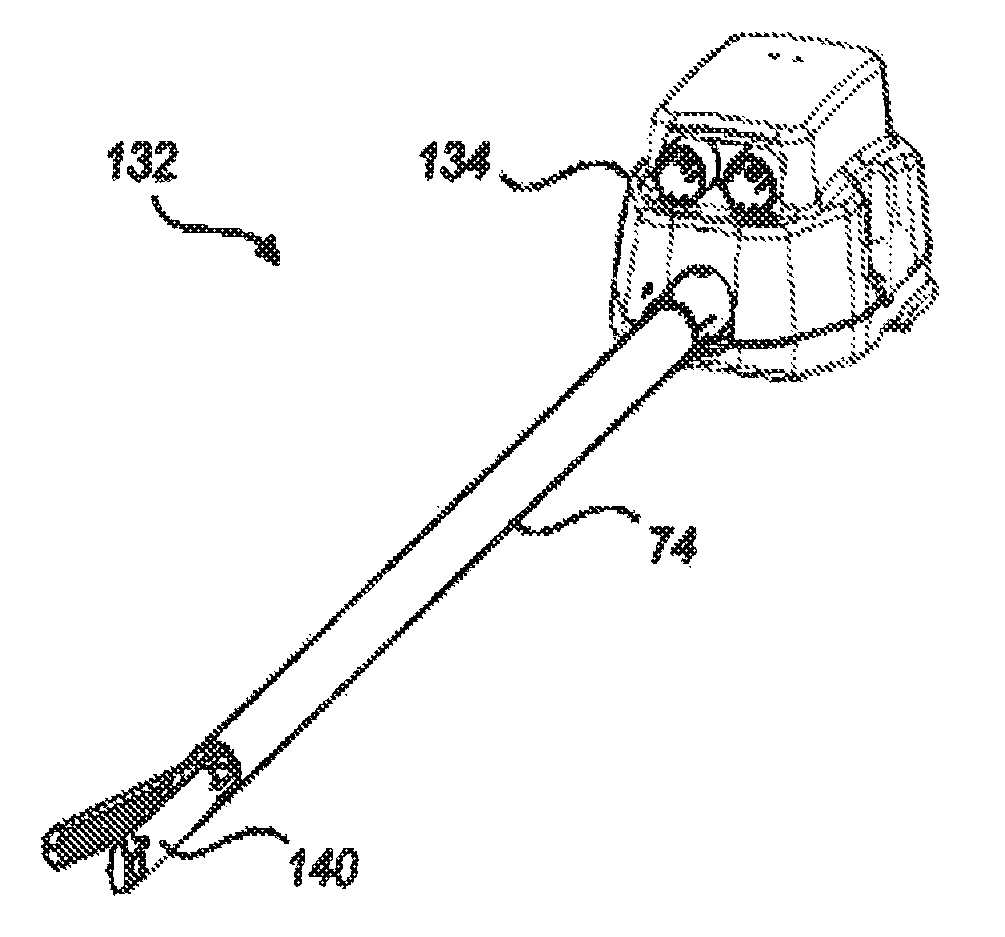
Motor interface for parallel drive shafts within an independently rotating member
ActiveUS8640788B2Force articulationIncrease clamping forceDrilling rodsConstructionsRotational axisDrive shaft
Owner:INTUITIVE SURGICAL OPERATIONS INC
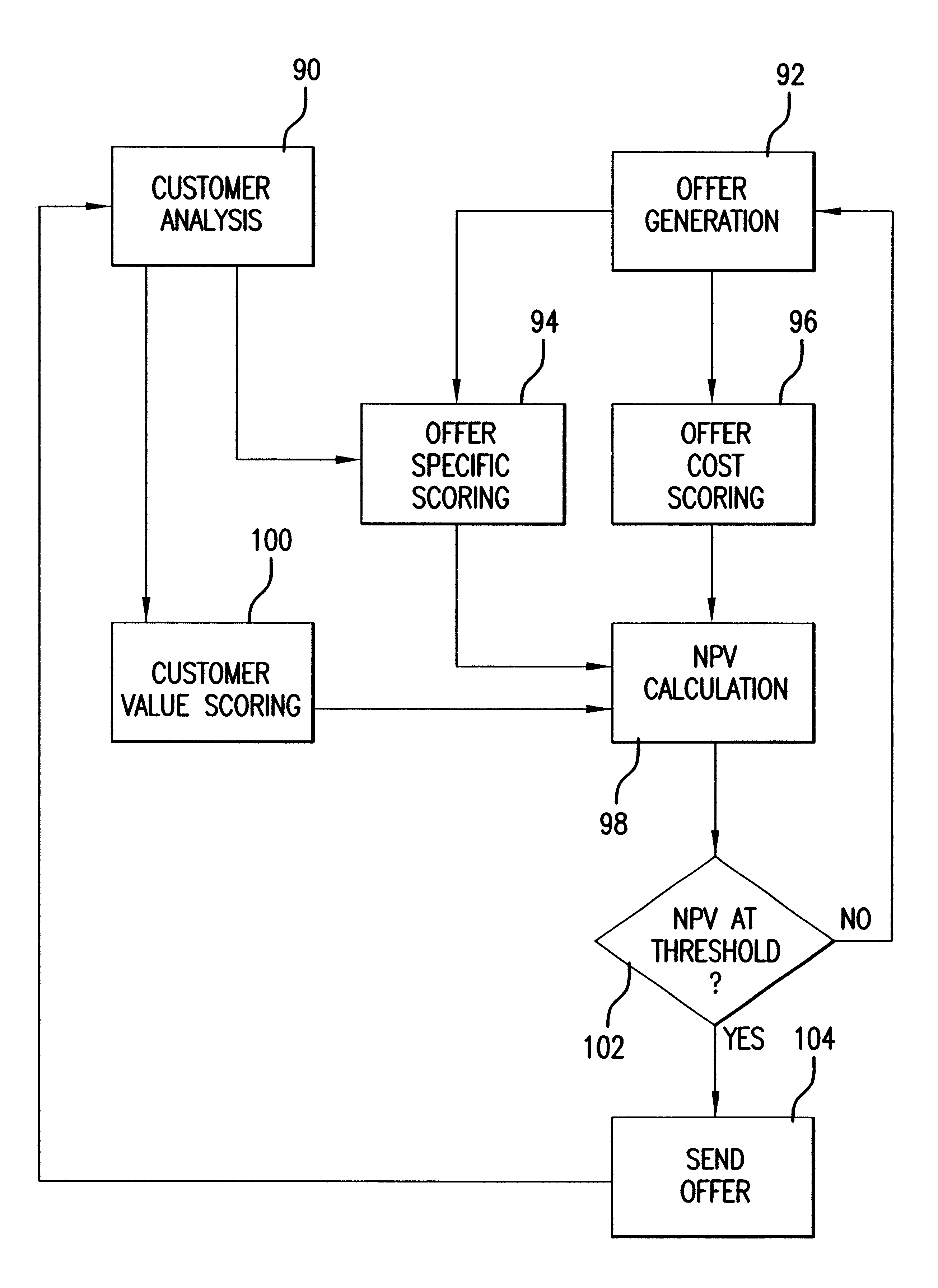
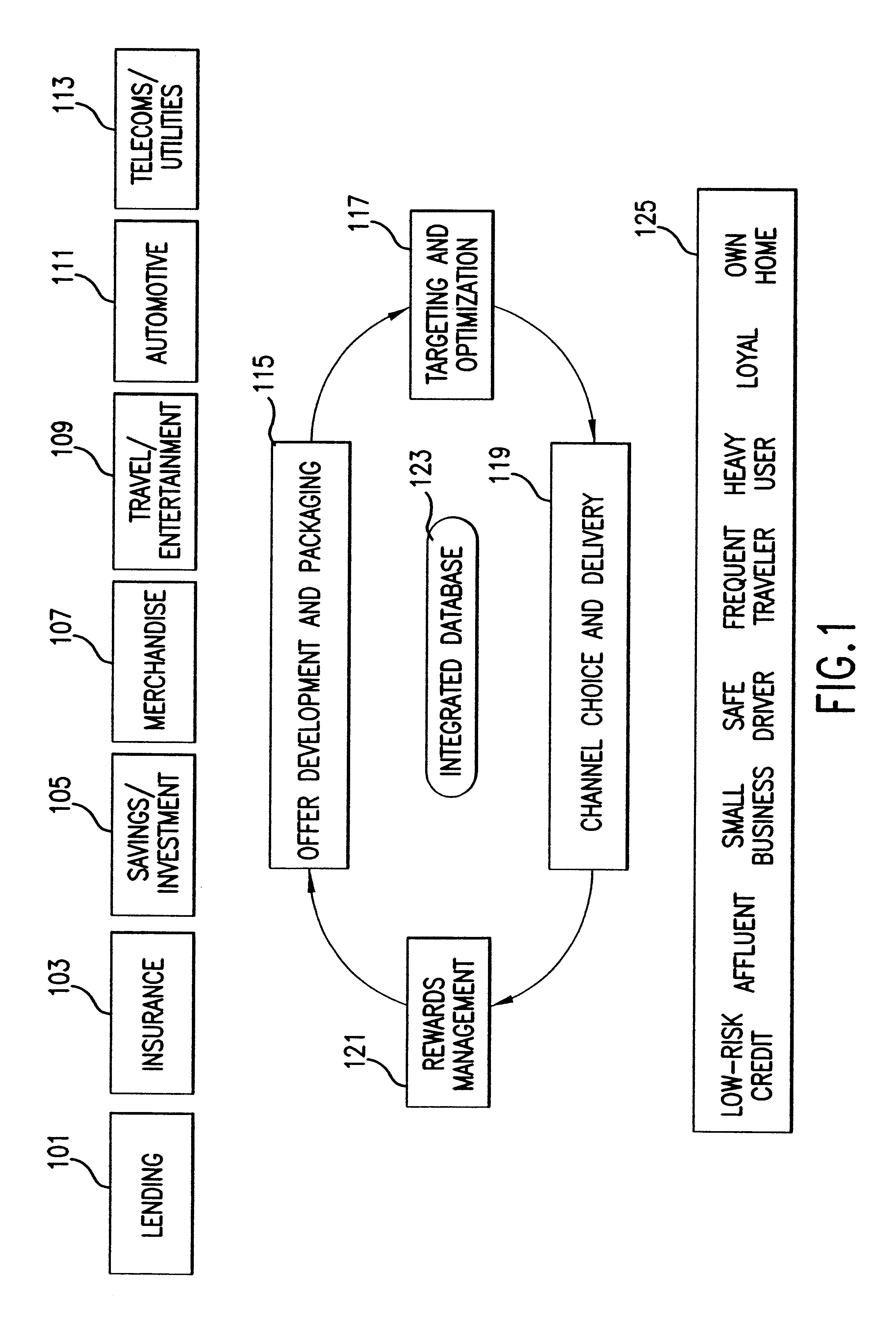
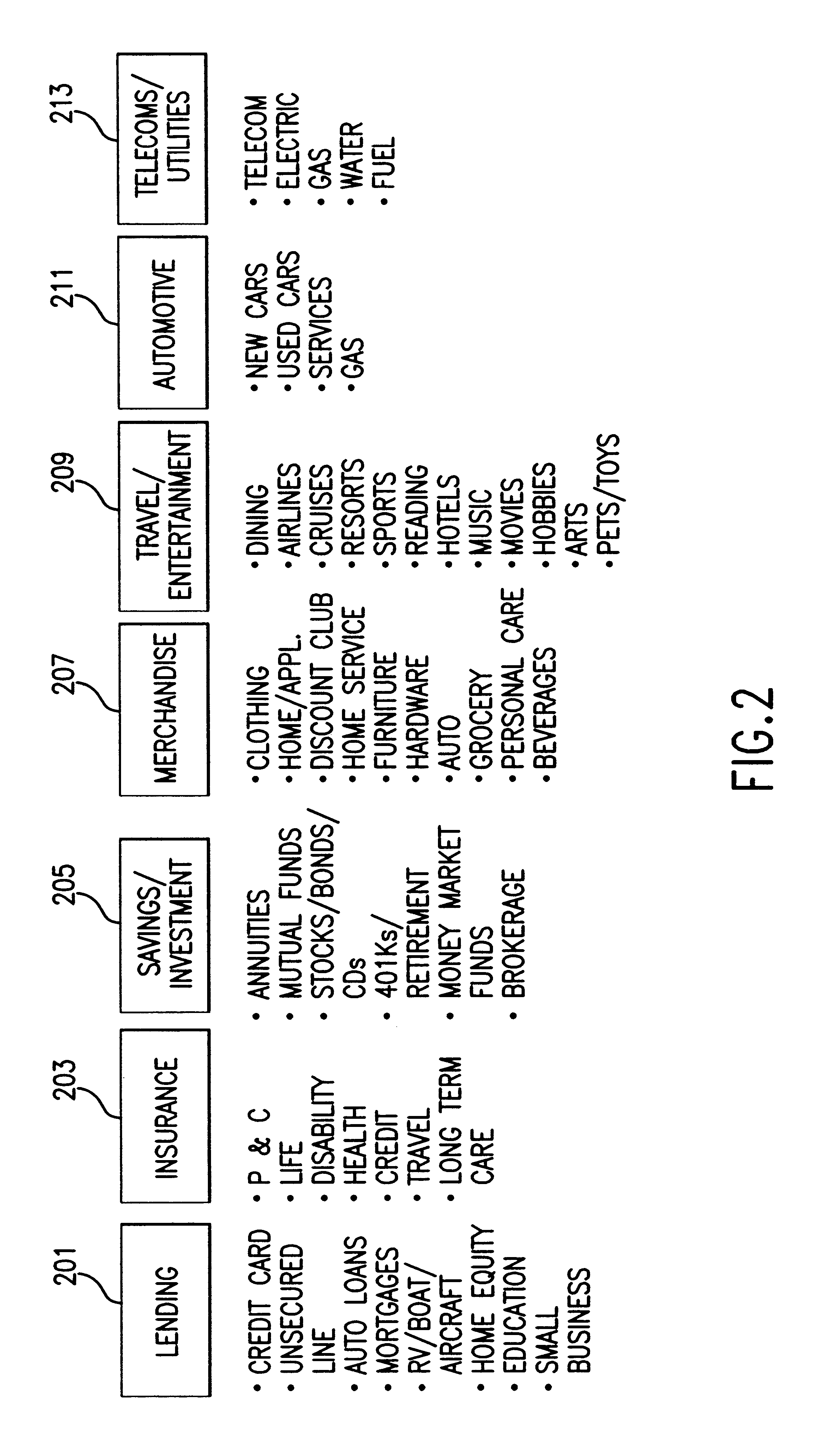
System and method of targeted marketing
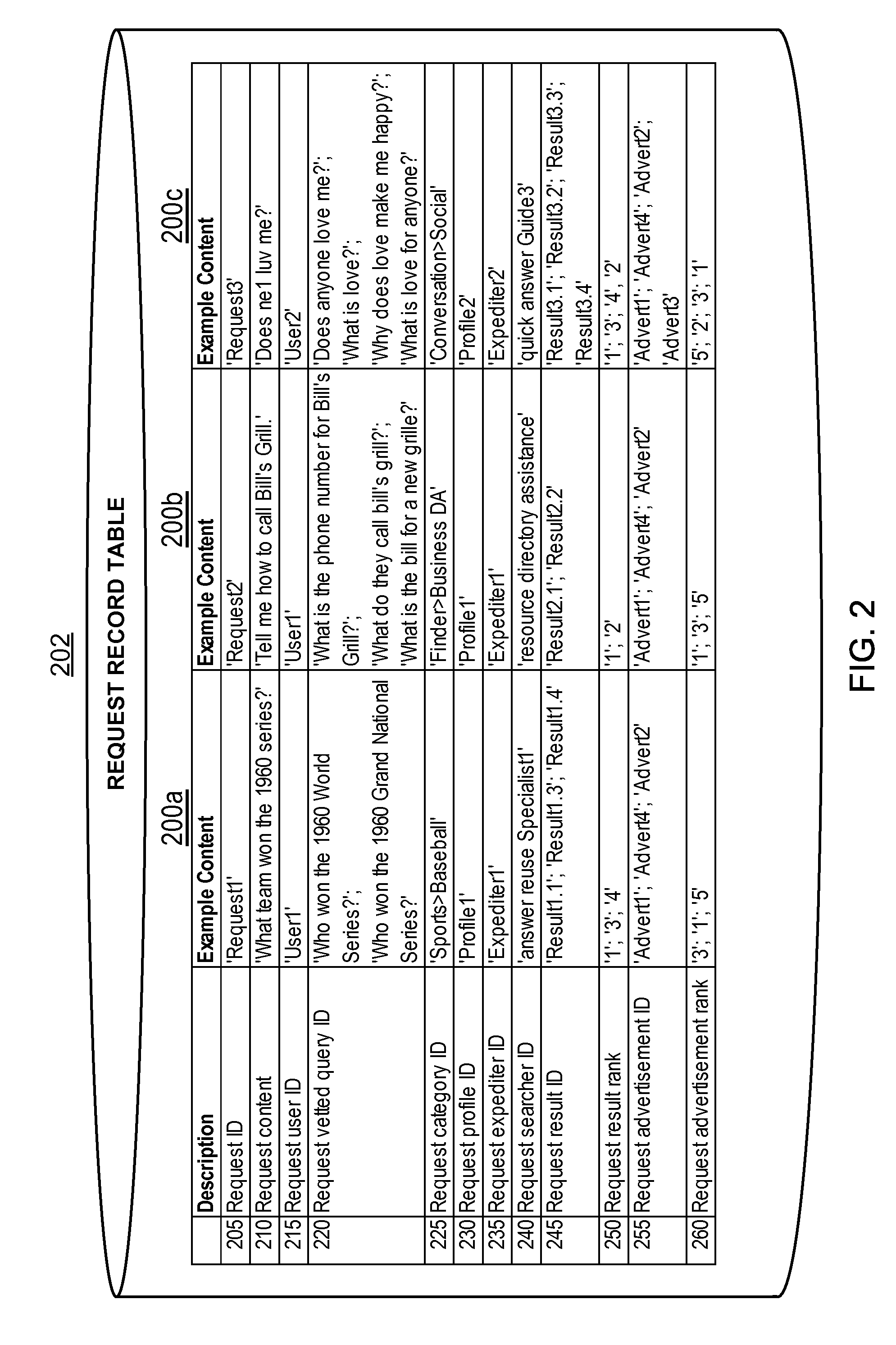
InactiveUS6925441B1High response rateDescribe wellMarket predictionsDiscounts/incentivesSocio economyValue creation
A system and method of targeted marketing to consumers, including businesses and associates, based upon the financial characteristics of the consumer, type offer being made and the channel of communication for delivery of the offer. The consumer is characterized based upon financial, behavioral, and socioeconomic factors. The offer is characterized based upon the consumer and the potential for the consumer accepting the offer. The channel of communication for delivery of the offer is also characterized and combined with the consumer and consumer-offer characteristics to arrive at a net present value of the offer to be made. If the net present value is sufficient the offer is processed and presented to the consumer. If the net present value is not sufficient, the offer is revised to present a better value to the consumer (or discarded if the required offer value can not be created) thereby enhancing the chances that the consumer will accept the offer in question. In this way the system and method of the target marketing creates value in both releasing, and not releasing, specific offers.
Owner:EXPERIAN INFORMATION SOLUTIONS
Combination therapy for depression, prevention of suicide, and various medical and psychiatric conditions
InactiveUS7973043B2Preventing disease progression/modifyingDelaying/preventing relapseBiocideNervous disorderInitial treatmentTherapy resistant
The present invention relates to a new method of treatment for persons meeting diagnoses for major depressive disorder, or other unipolar (non-bipolar, non-psychotic and non-treatment resistant) depression. The method comprises administering a combination of two categories of drugs, antipsychotics or dopamine system stabilizers, in combination with a newer antidepressant such as a selective serotonin reuptake inhibitor, as initial treatment or as soon as possible. The method targets the prevention of suicide, and provides other benefits including preventing disease progression development of tolerance toward the antidepressants. Another aspect of the invention relates to using the method for alleviating cognitive distortion and related functional impairment or health risks, and / or using the method for smoking cessation or nicotine withdrawal.
Owner:MIGALY PETER
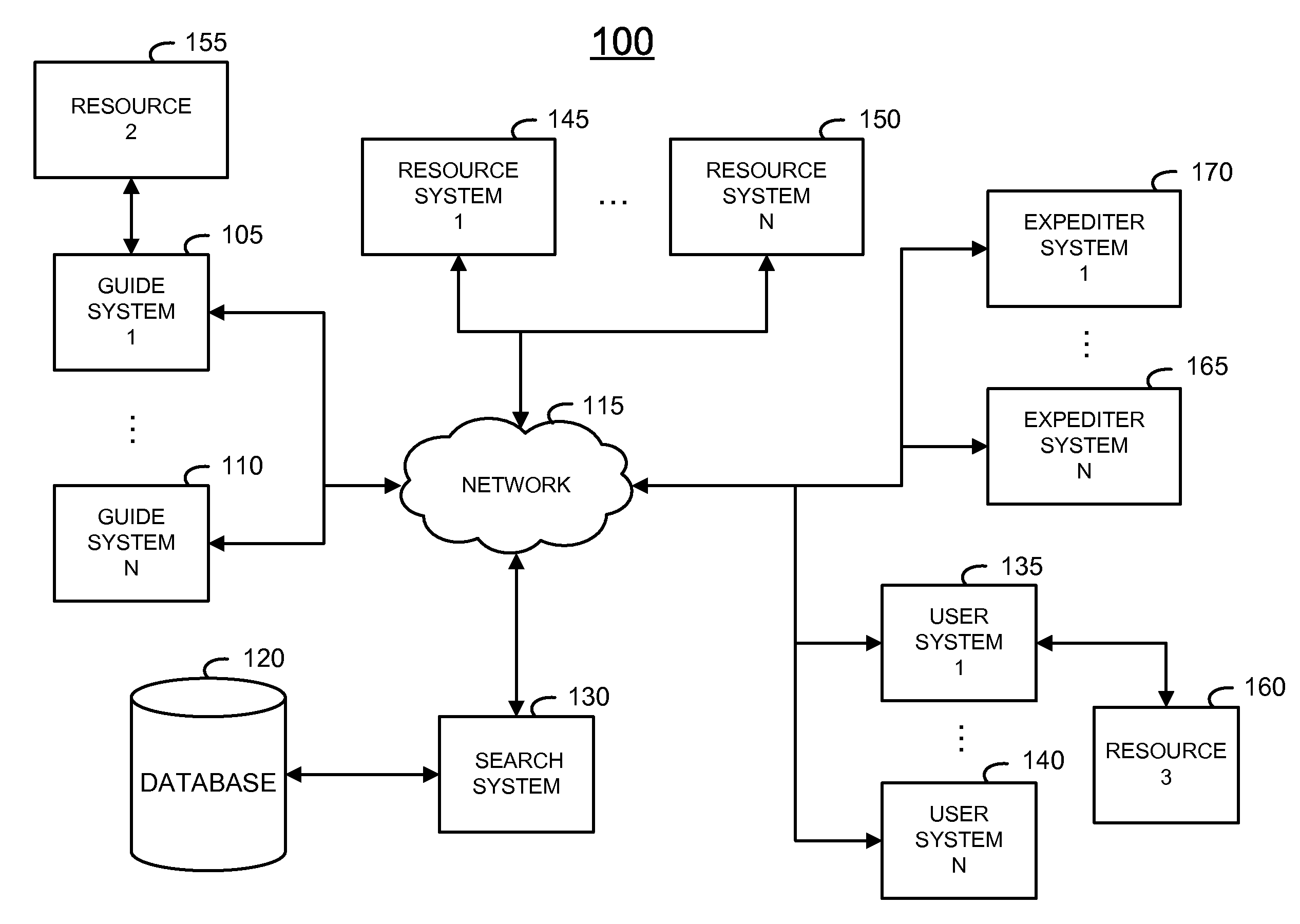
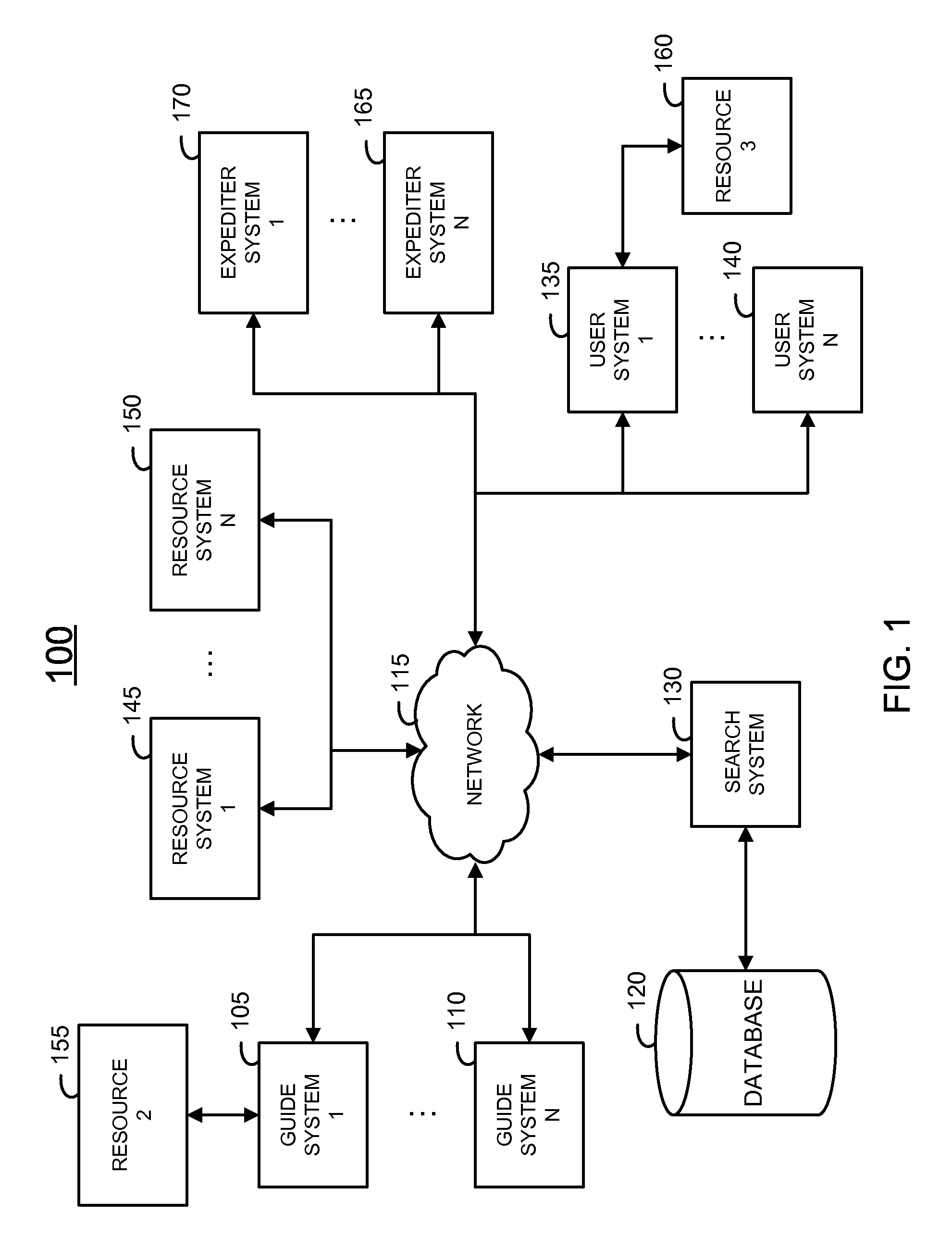
Method and system of providing a search tool
InactiveUS20110010367A1High response rateDigital data information retrievalDigital data processing detailsData miningRapid response
A system and method of providing information to improve efficiency of human searchers obtaining information on behalf of users is described. A first responder to a request is provided with specialized tools for responding to a request. An initial evaluation of a query may be used to determine the responder and the type of tools which will be provided initially. A toolset which allows rapid responses based on data resources, common queries, and contextual information of a user, a request, partially matching queries and previous responses to previous queries is used to improve the probability that a first responder may provide a suitable response to a query.
Owner:CHACHA SEARCH
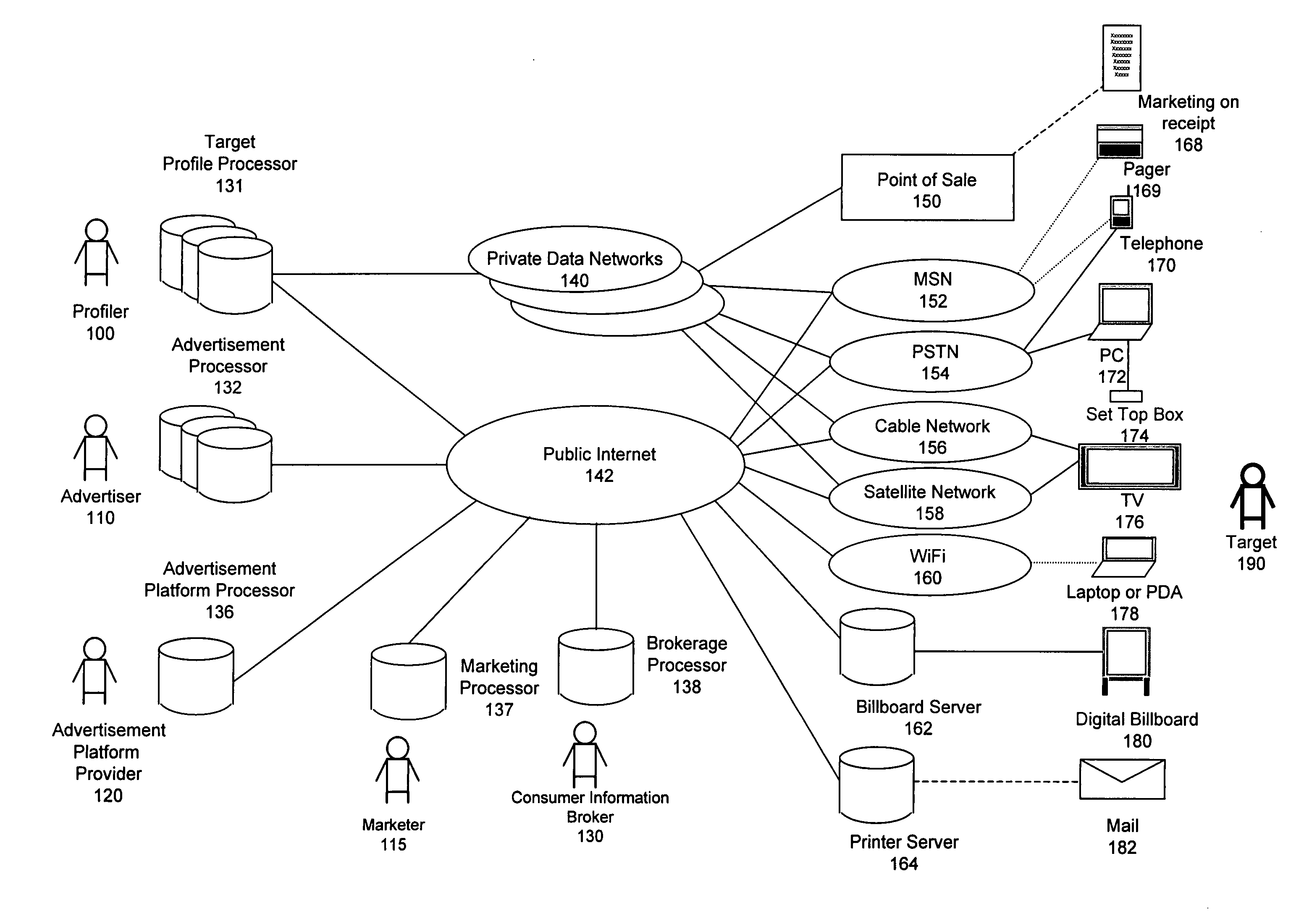
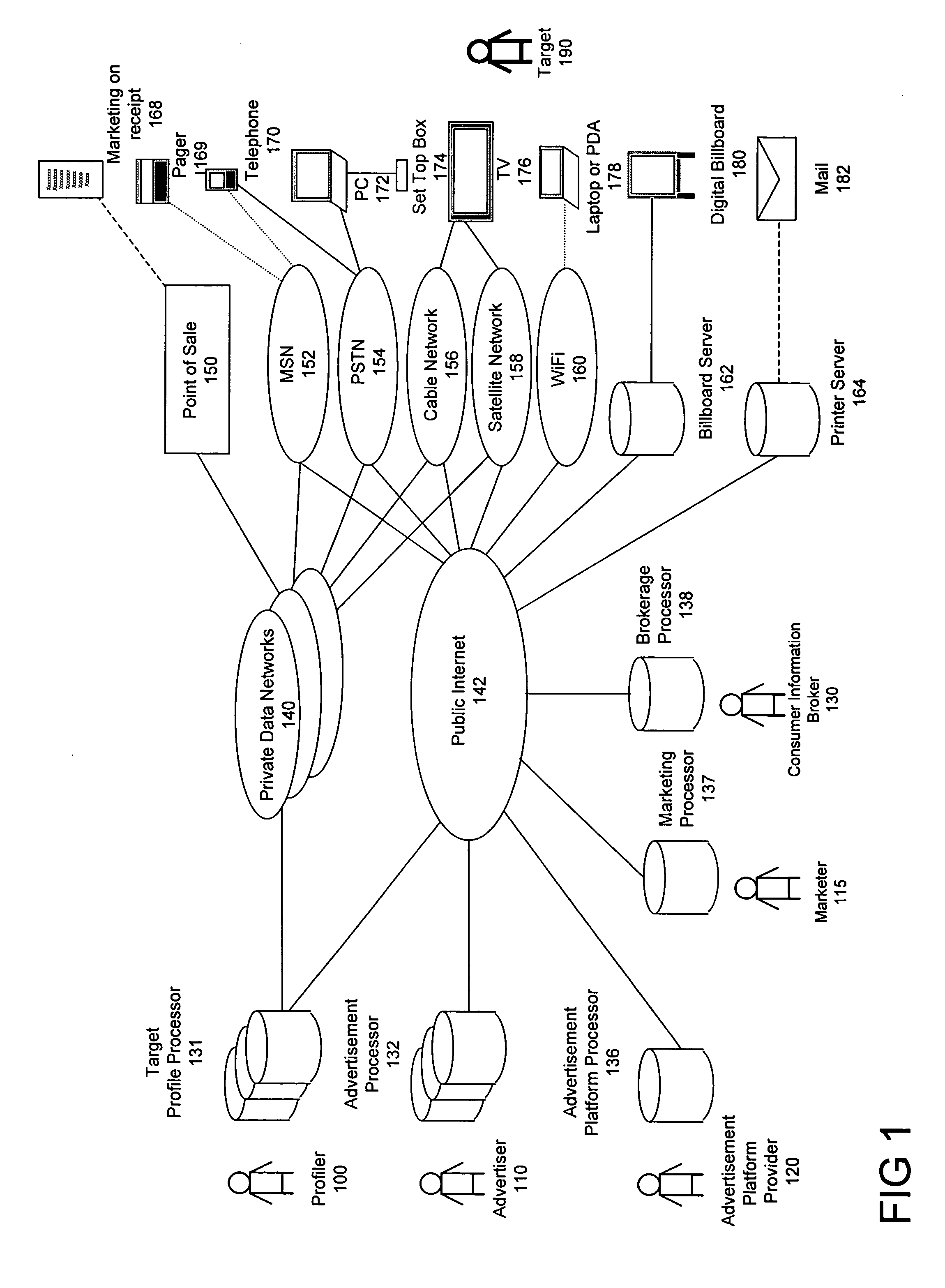
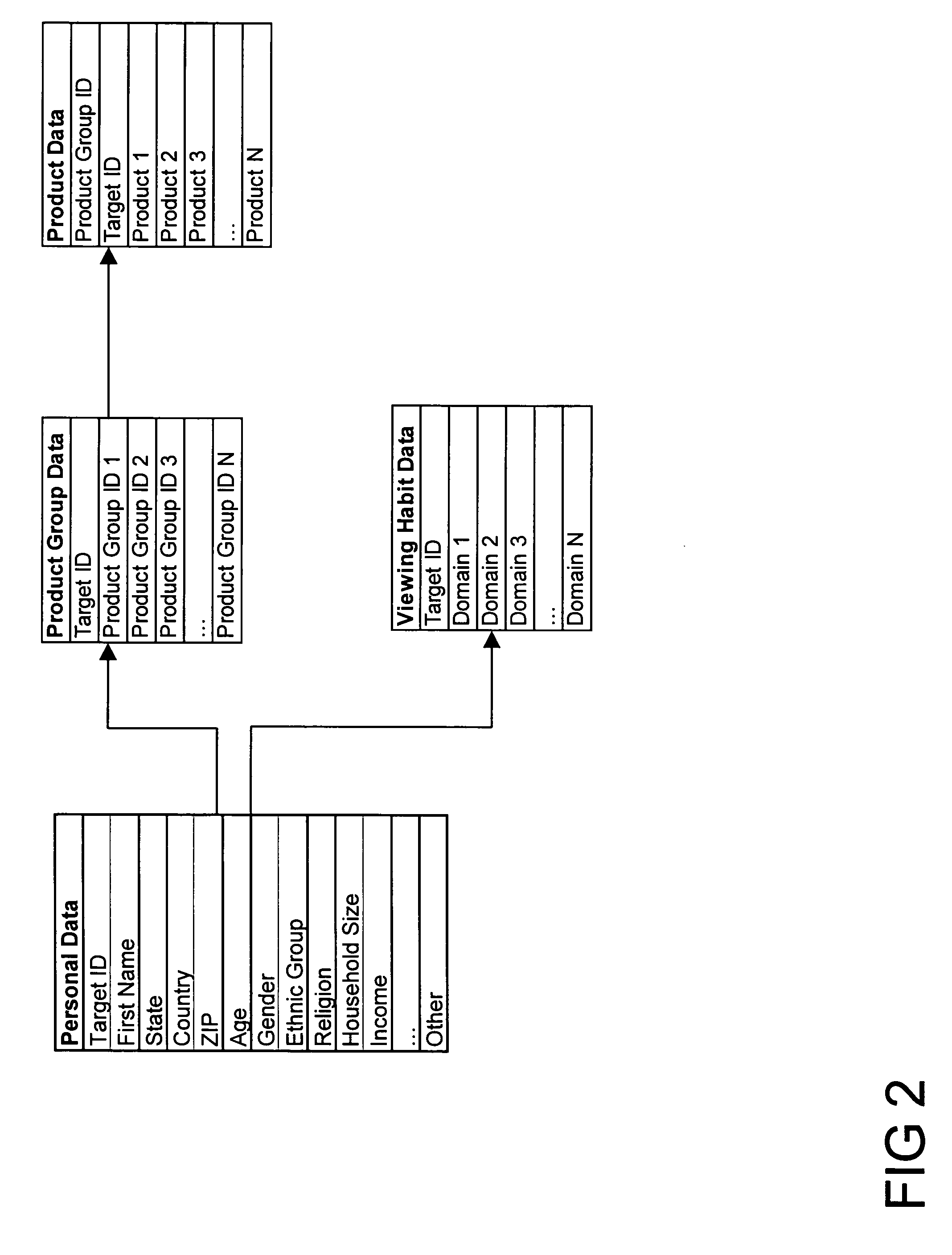
Method for consumer data brokerage
The present invention provides a method to broker consumer data for the purpose of marketing activities and to compensate consumers for the use of their data. The method provides mechanisms and processes for consumer information brokers to allow providers of real-time advertisement platforms or any static advertisement platform to offer advertisers the opportunity to dynamically bid on individual direct to consumer advertisement opportunities and to adapt individual advertisements according to the profile of each consumer. Furthermore the method allows consumer information brokers to offer marketers targeted direct to consumer marketing measures based on well-defined target profiles. Consumers have the opportunity to allow advertisers to convey advertisements to them and be compensated for viewing them. They may also opt out and forego the opportunity. Furthermore consumers have the opportunity to participate in direct to consumer marketing measures and be compensated for participating. This will lead to significantly improved return on investment for businesses and help them to develop products and services that consumers really want.
Owner:JASCHKE MICHAEL
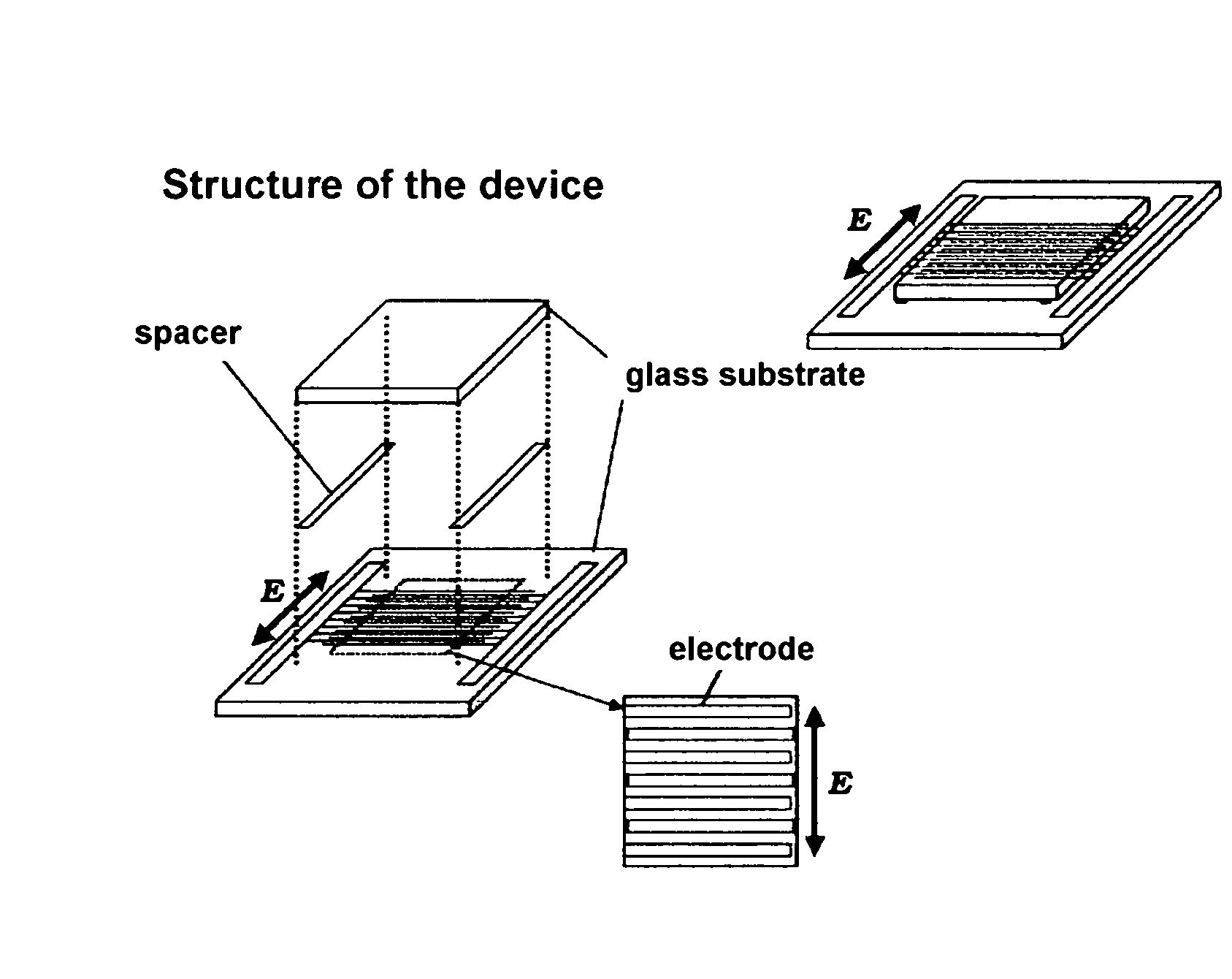
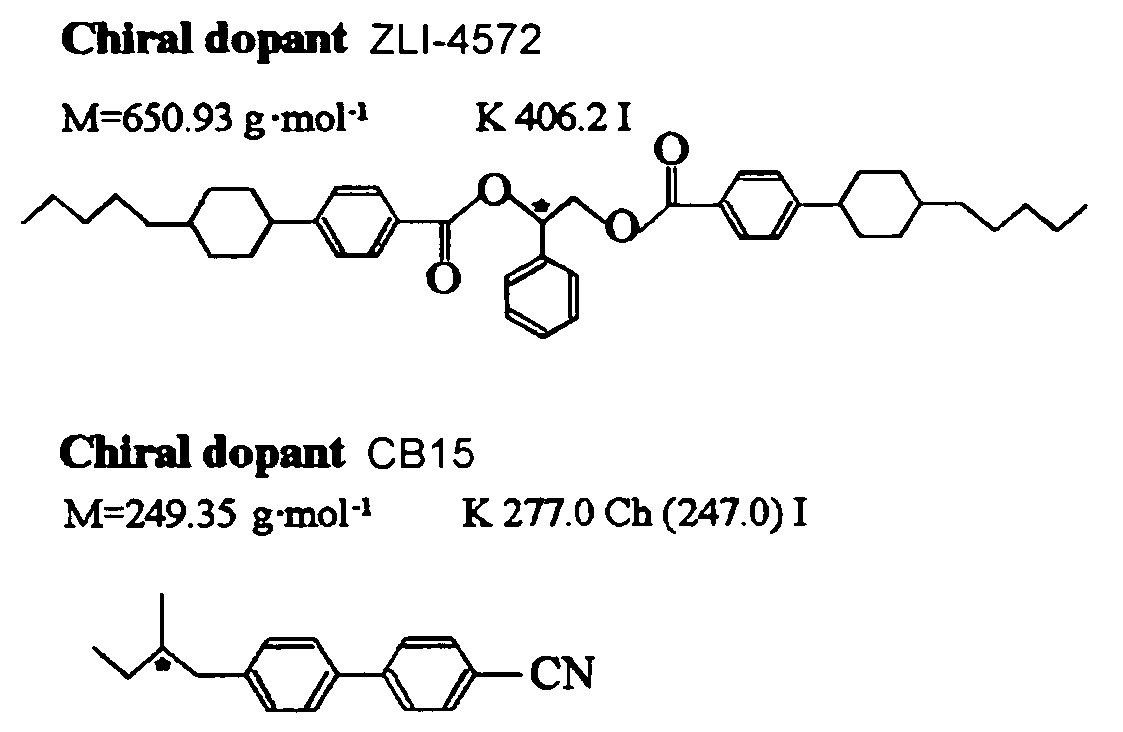
Liquid Crystal Display Device
ActiveUS20080259254A1High response rateQuality improvementLiquid crystal compositionsNon-linear opticsIn planeDopant
[PROBLEMS] To provide a liquid crystal display device that does not need any surface aligning treatment, realizes striking increase of a response speed of movie display and is free from any light leakage (produce dark field) at black display.[MEANS FOR SOLVING PROBLEMS] There is provided a liquid crystal display device comprising a pair of transparent substrates and, interposed therebetween, a polymer stabilized blue-phase liquid crystal. The liquid crystal display device utilizing the polymer stabilized blue-phase liquid crystal exhibits a large birefringence change upon application of an electric field to cell substrate in an in-plane direction. The polymer stabilized blue-phase liquid crystal is composed of a low-molecular liquid crystal capable of developing a blue phase between cholesteric phase and isotropic phase and a polymer network created in the low-molecular liquid crystal. Further, by optimizing the type and amount of chiral dopant added to the liquid crystal, there can be provided a liquid crystal display device that is free from any light leakage (produce dark field) at black display.
Owner:JAPAN SCI & TECH CORP
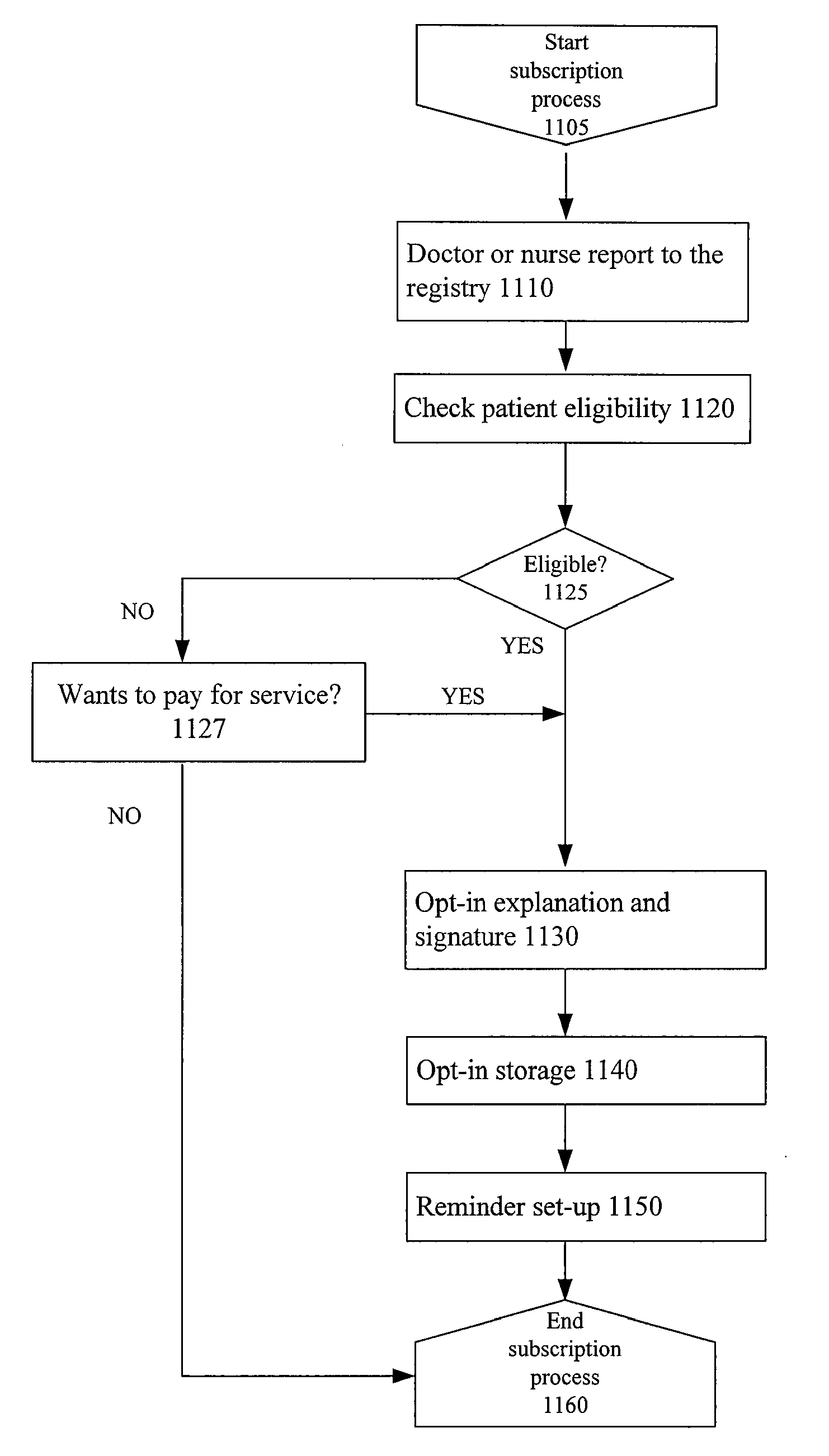
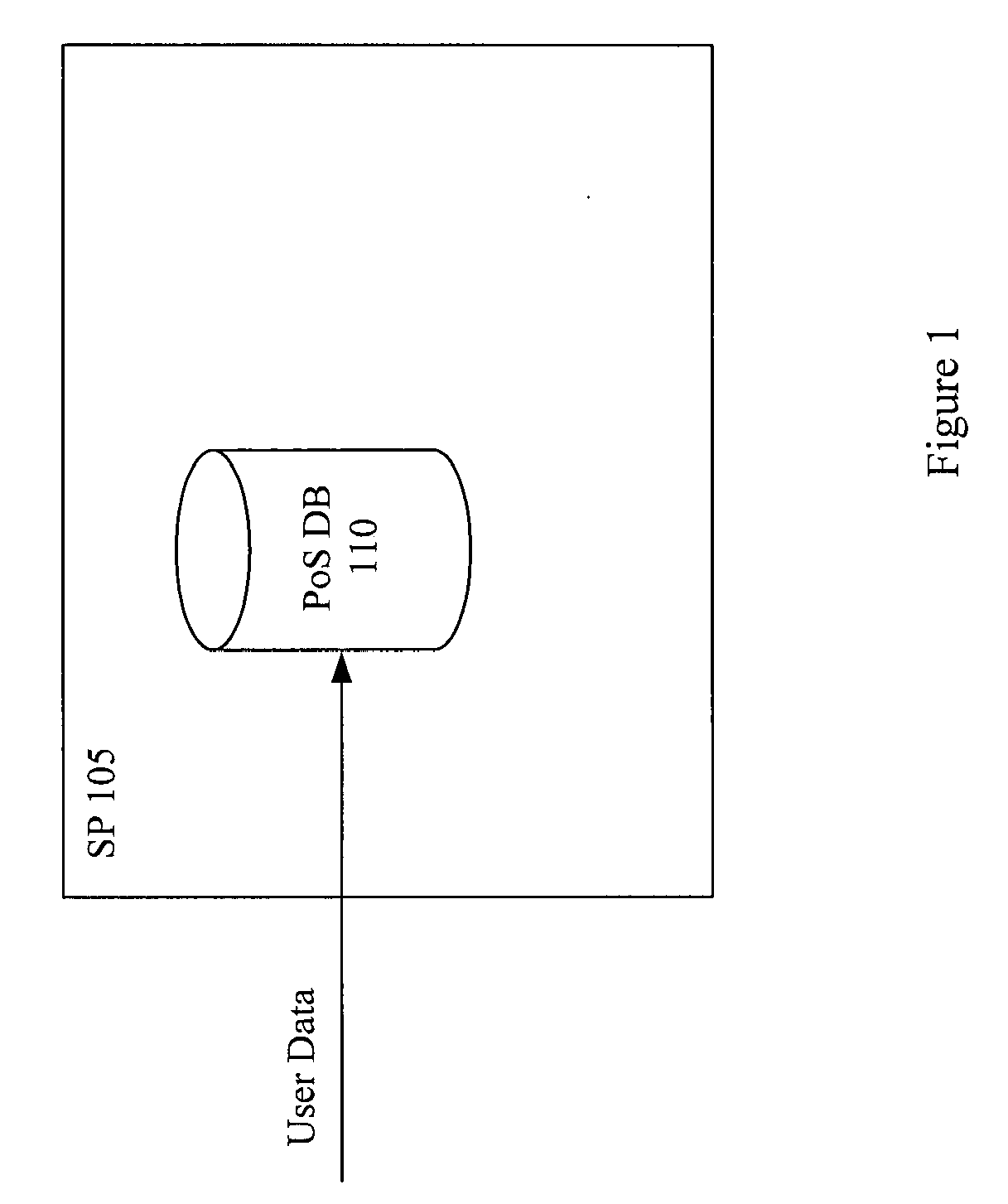
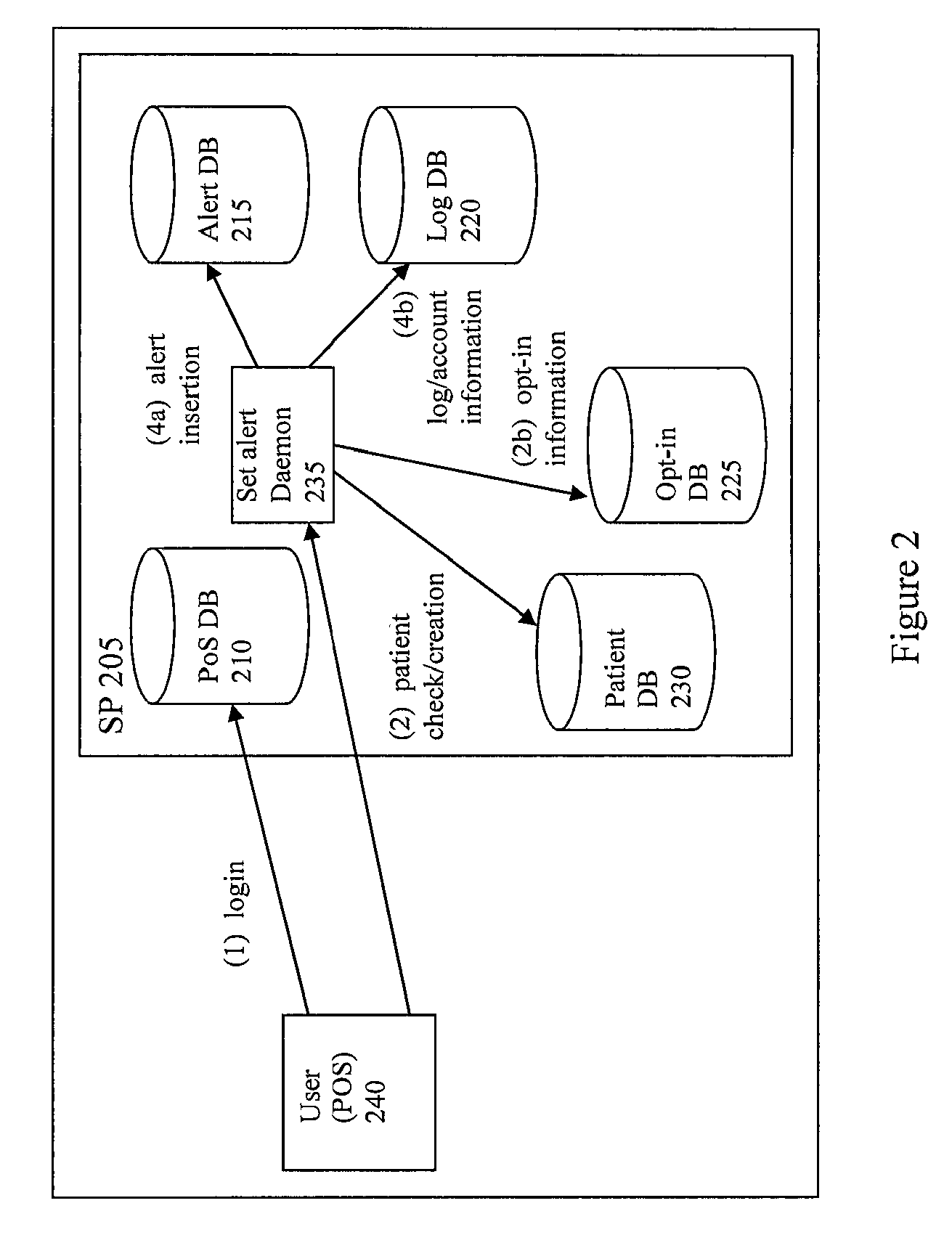
Systems and Methods To Improve The Efficiencies Of Immunization Registries
InactiveUS20080177574A1Value maximizationStrengthen linkData processing applicationsComputer-assisted medical data acquisitionPaymentCaregiver person
Systems and methods are provided for setting up and sending healthcare alerts (e.g. immunization reminders) to patients / caregivers through the use of communications devices. In an embodiment, information about a patient's / caregiver's wireless device (e.g. cell phones) is collected and reported to an immunization registry. The registry is linked to a system that uses the wireless device information to generate automatic wireless immunization reminders, recalls and educational messages that are sent to the patient / caregiver. In a preferred embodiment, opt-in information is reported to the system to ensure that the patient / caregiver has consented to the automatic wireless immunization reminders. The system may also check the eligibility of patients to receive reminders, provide incentives for patients to receive reminders, provide follow up information (e.g. performed, not performed, responses to message / s) to a healthcare provide for a particular patient or a group of patients, and / or provide billing services for payment of the reminders.
Owner:LARA GONZALEZ MARCOS +3
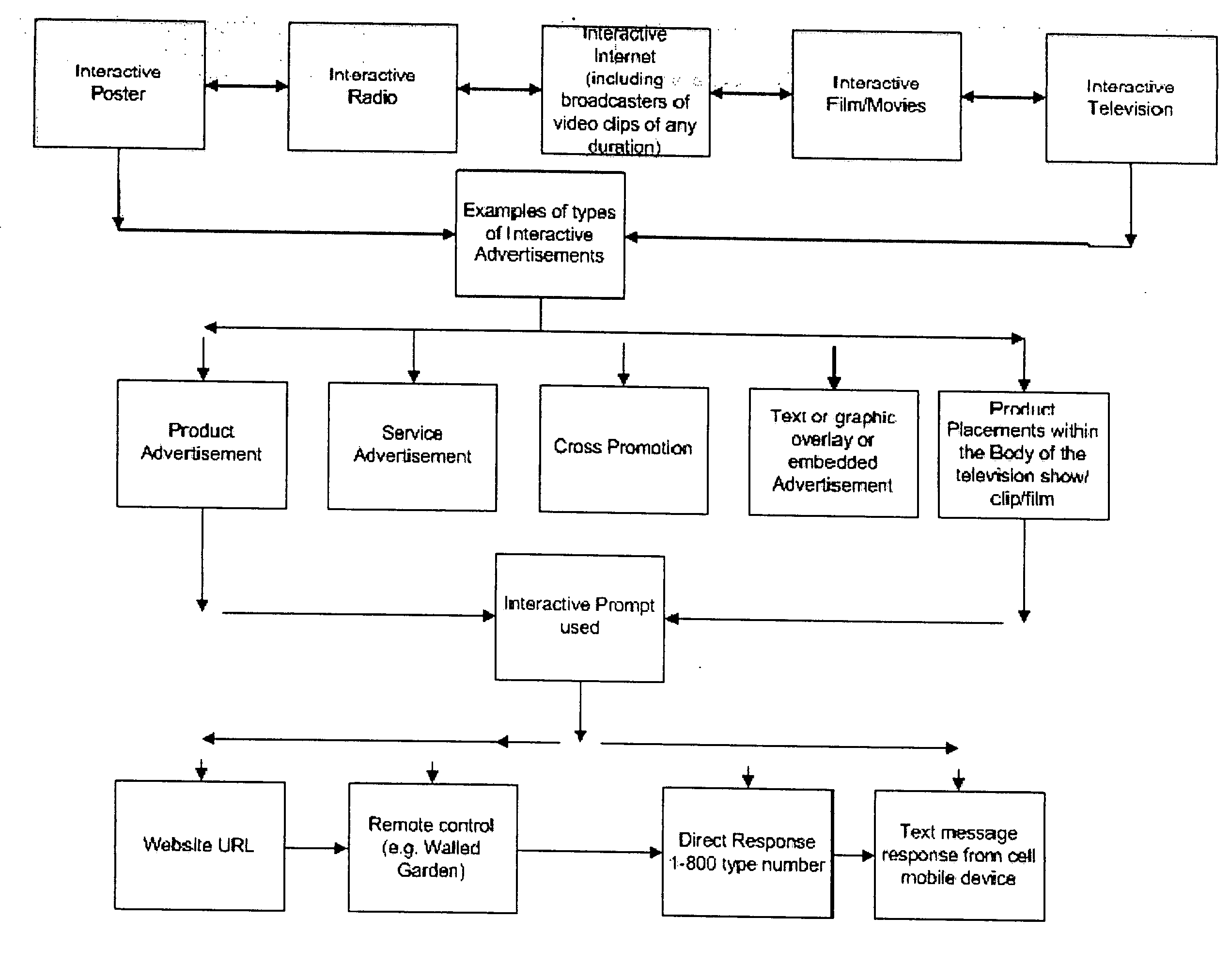
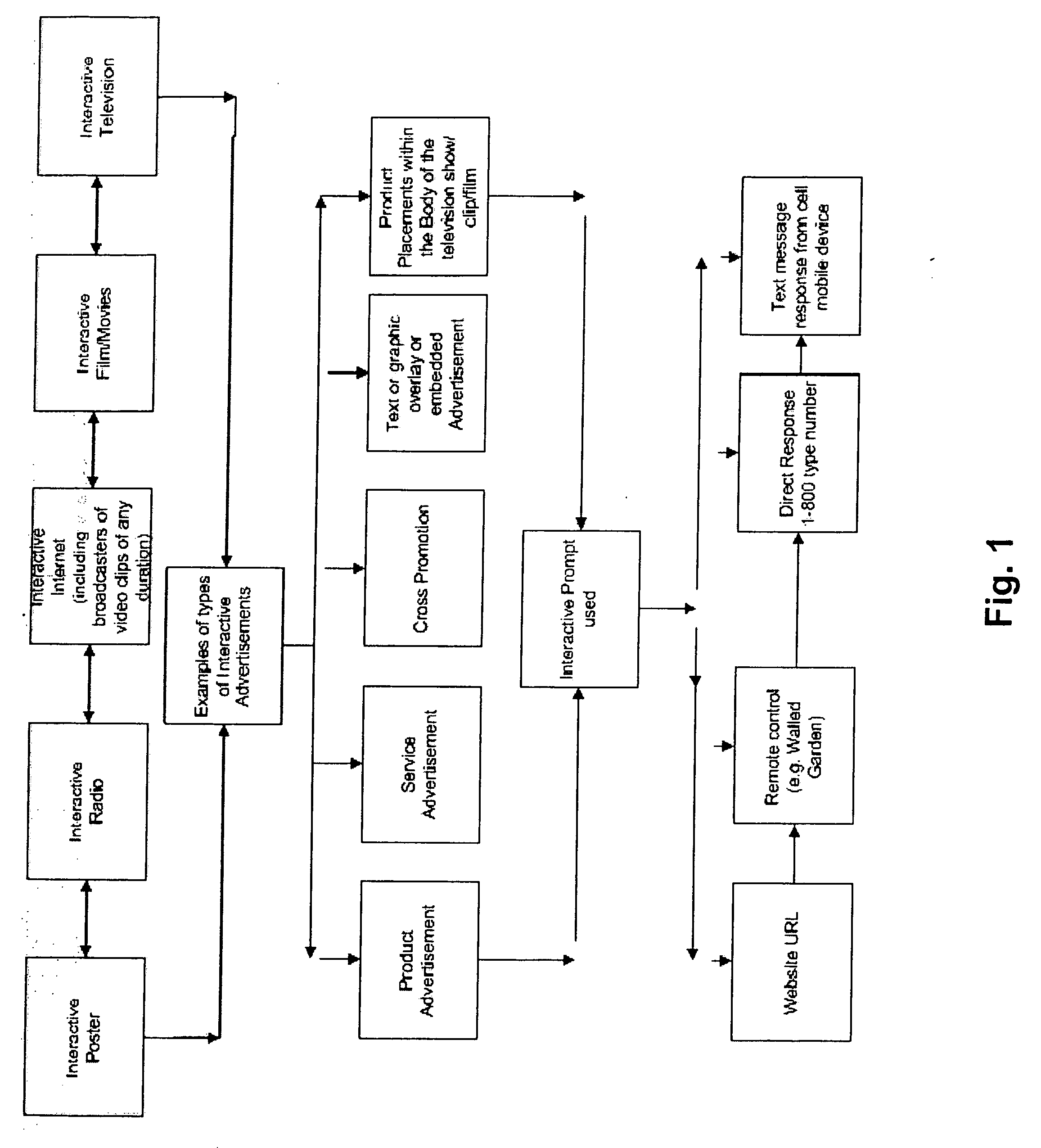
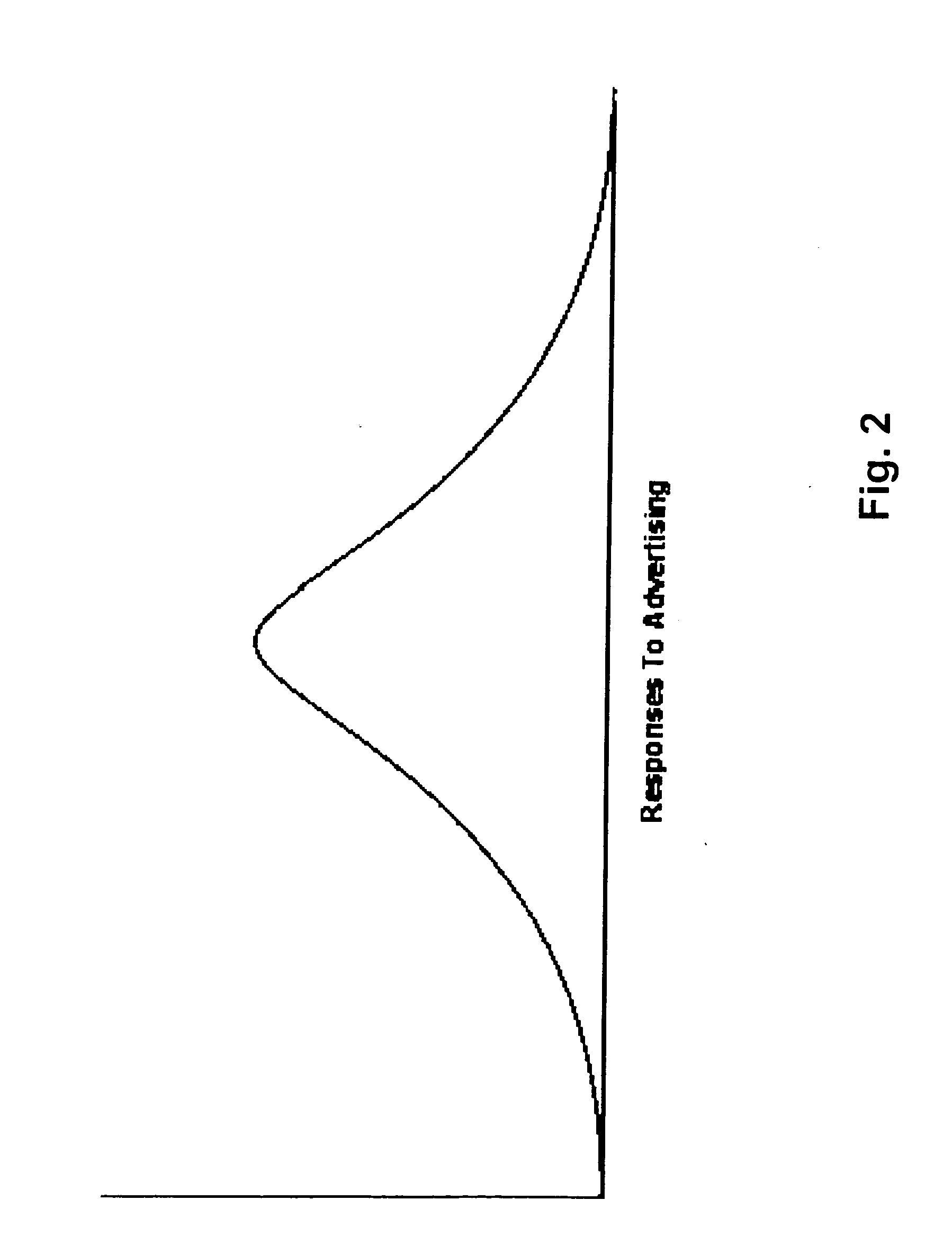
Method for determining, correlating and examining the causal relationships between media program and commercial content with response rates to advertising and product placement
ActiveUS20090210290A1Promote resultsHigh response rateDiscounts/incentivesAnalogue secracy/subscription systemsCausalitySpecific time
A method of determining correlations and causality between media program content and consumer responsiveness involves identifying and storing media and commercial program time occurrence and content information and consumer media reviewing actions which occur in connection with the media and commercial program time occurrence and content information. The information is correlated to obtain and assign responsiveness probability values corresponding to type and intensity of consumer response for each of the media and commercial program time occurrence and content information. These responsiveness probability values are then applied to a second media program to place product advertising at a specific time within specific content therein as determined by the responsiveness probability values thus facilitating creation of new ads and modification of existing ones and further, directing placement of those advertisements within any and all broadcast and Internet media programming.
Owner:ELLIOT SEBASTIAN
Therapeutic agent for hepatitis C
InactiveUS9006285B2High response rateImprove securityBiocidePeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
Disclosed is a novel therapeutic means against interferon-resistant hepatitis C. Specifically disclosed are: a pharmaceutical composition for treating interferon-resistant hepatitis C, which is characterized by comprising at least one component selected from the group consisting of an ω-3 polyunsaturated fatty acid, a pharmaceutically acceptable salt of the fatty acid and an ester of the fatty acid as an active ingredient; and a method for utilizing the pharmaceutical composition.
Owner:MOCHIDA PHARM CO LTD
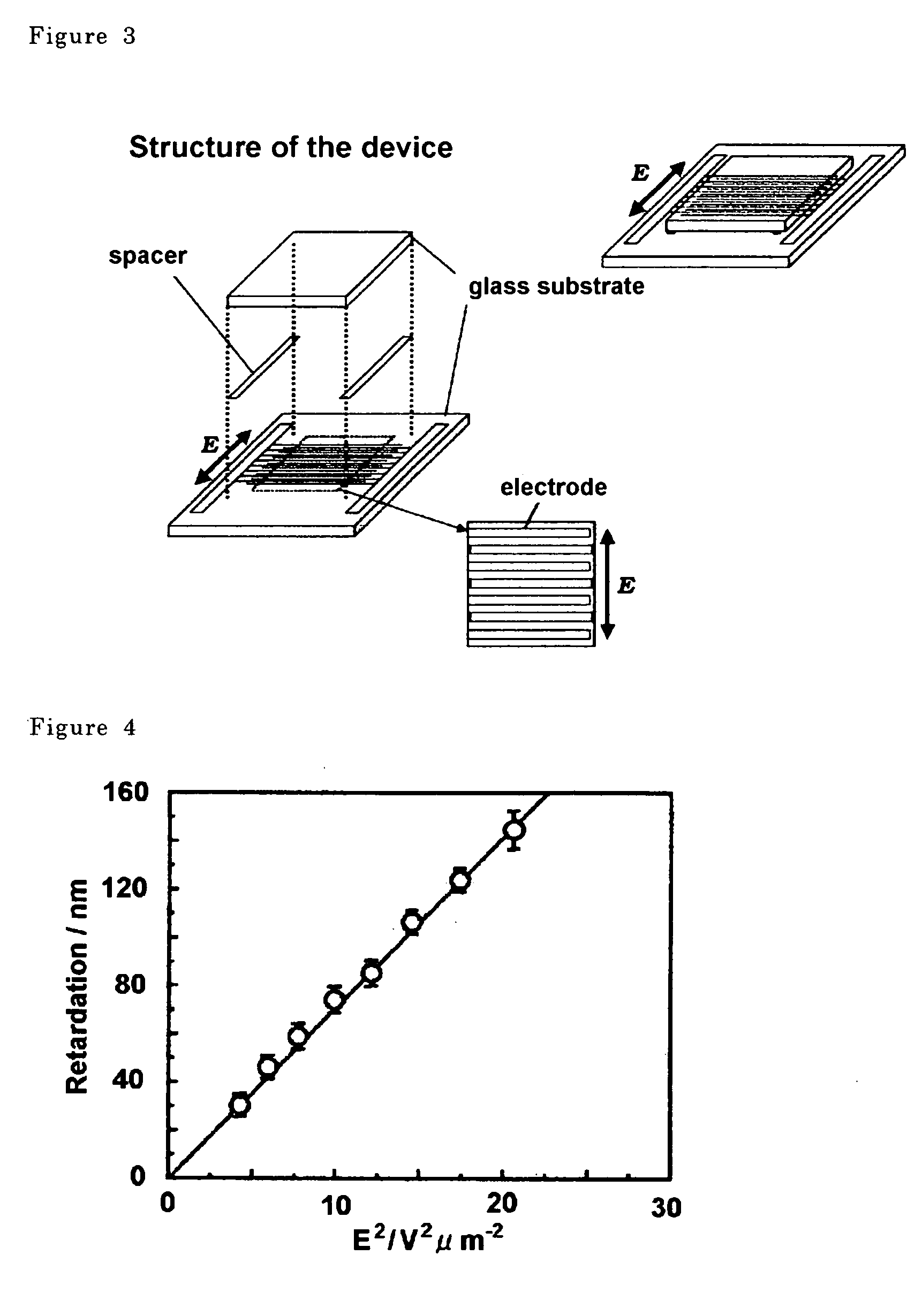
Liquid crystal display device
ActiveUS7576829B2High response rateQuality improvementLiquid crystal compositionsNon-linear opticsIn planeDopant
Presents a liquid crystal display device that does not require a surface orientation treatment, dramatically improves the response rate of dynamic image displays and does not experience light leaks when the display is black, which means to yield a dark field of vision. The liquid crystal display device comprises polymer-stabilized blue phase liquid crystals sandwiched between a pair of clear substrates. The liquid crystal display device obtained using polymer-stabilized blue phase liquid crystals exhibits large double refraction changes when an electrical field is applied in an in-plane direction to the cell substrates. The polymer-stabilized blue phases liquid crystals comprises a low molecular weight liquid crystal that allows a blue phase to appear between cholesteric and isotropic phases and polymer network formed in the low molecular weight liquid crystals. Furthermore, a liquid crystal display device that does not leak light when the display is black (yields a dark field of vision) can be obtained by optimizing the type and amount of a chiral dopant added to the liquid crystals.
Owner:JAPAN SCI & TECH CORP
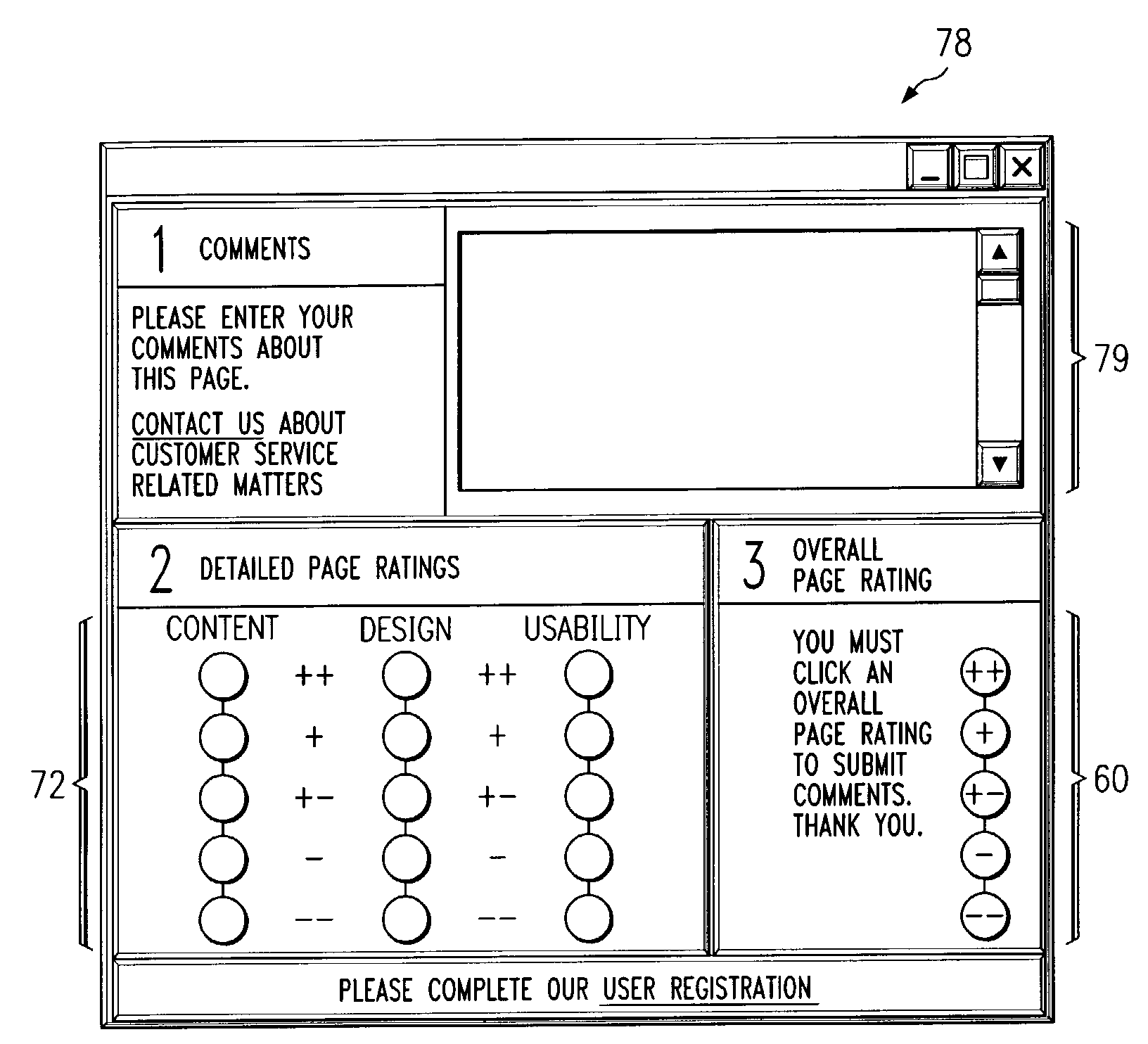
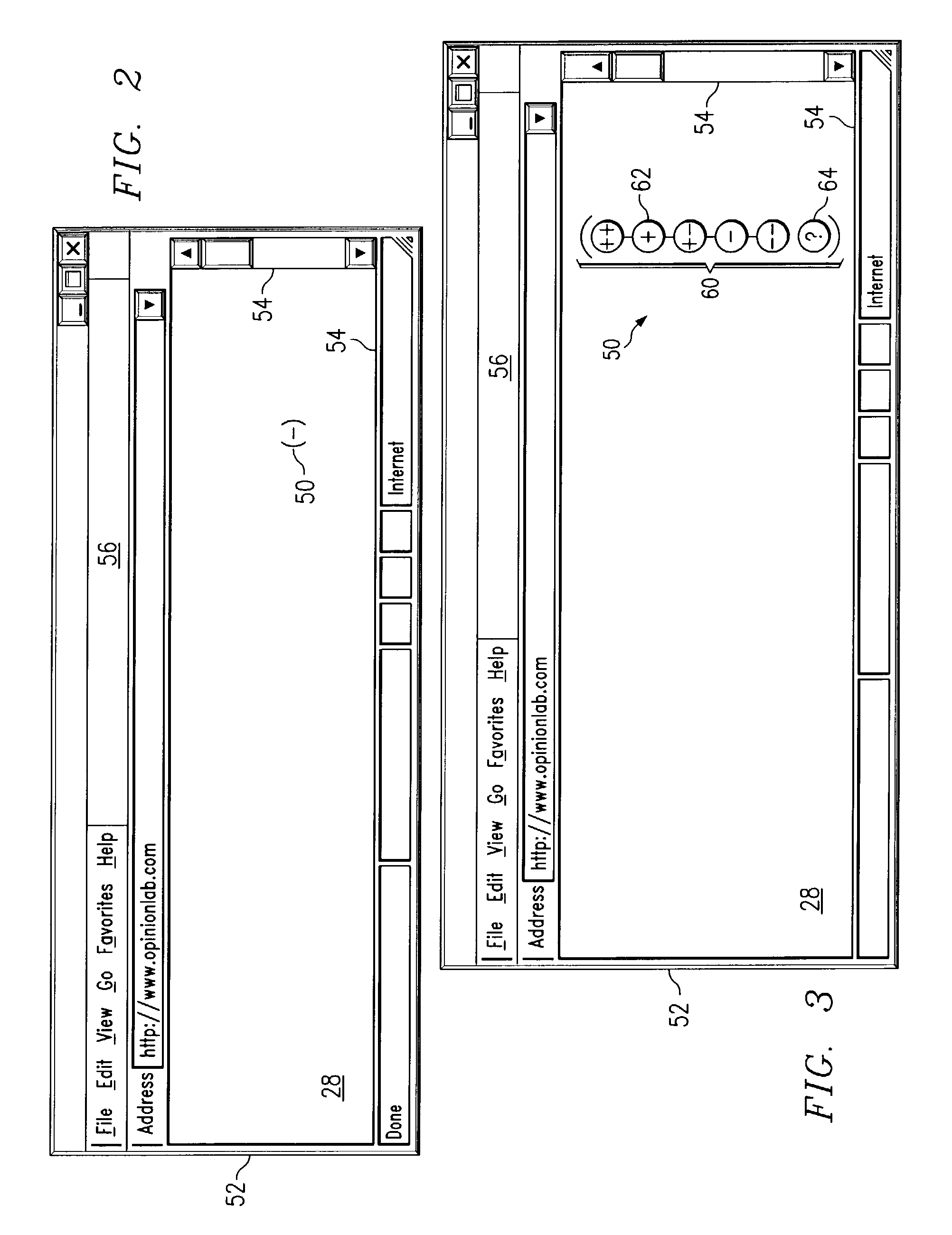
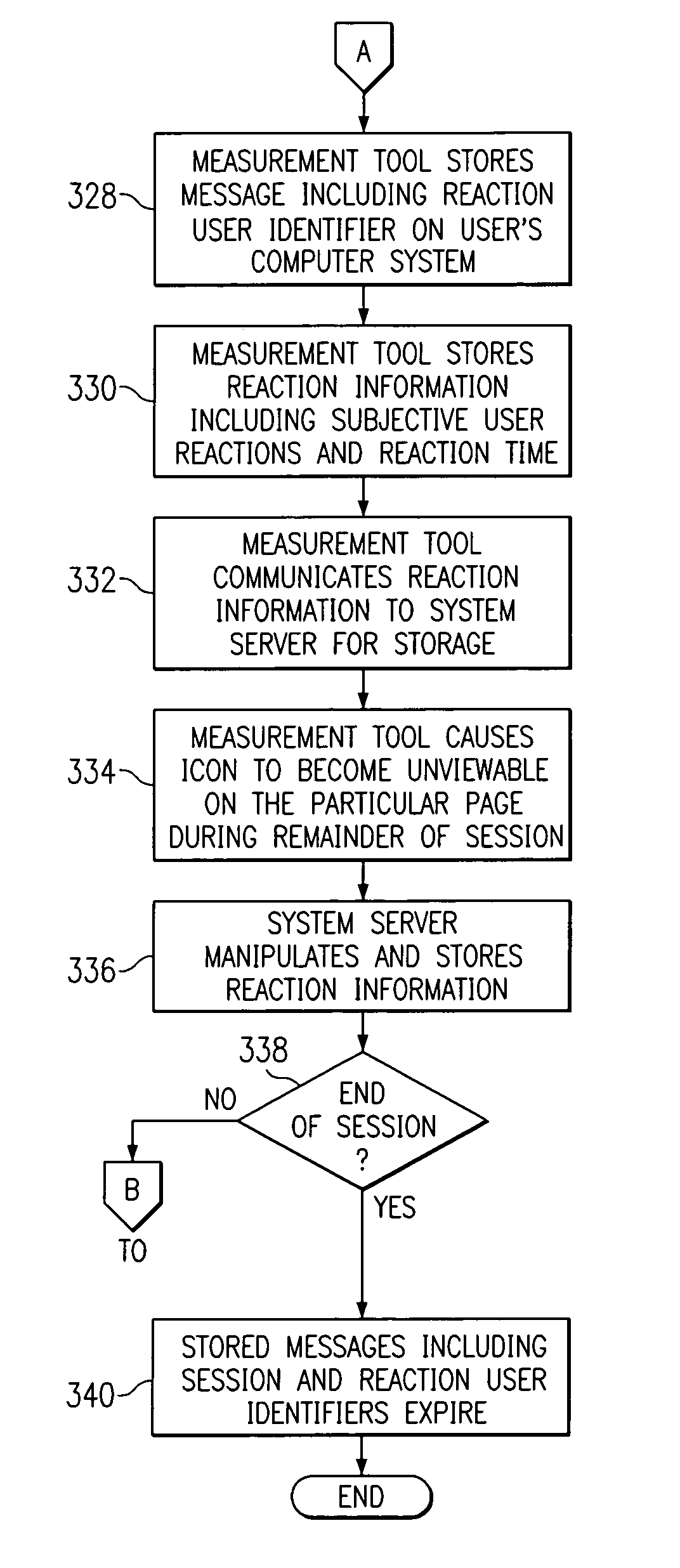
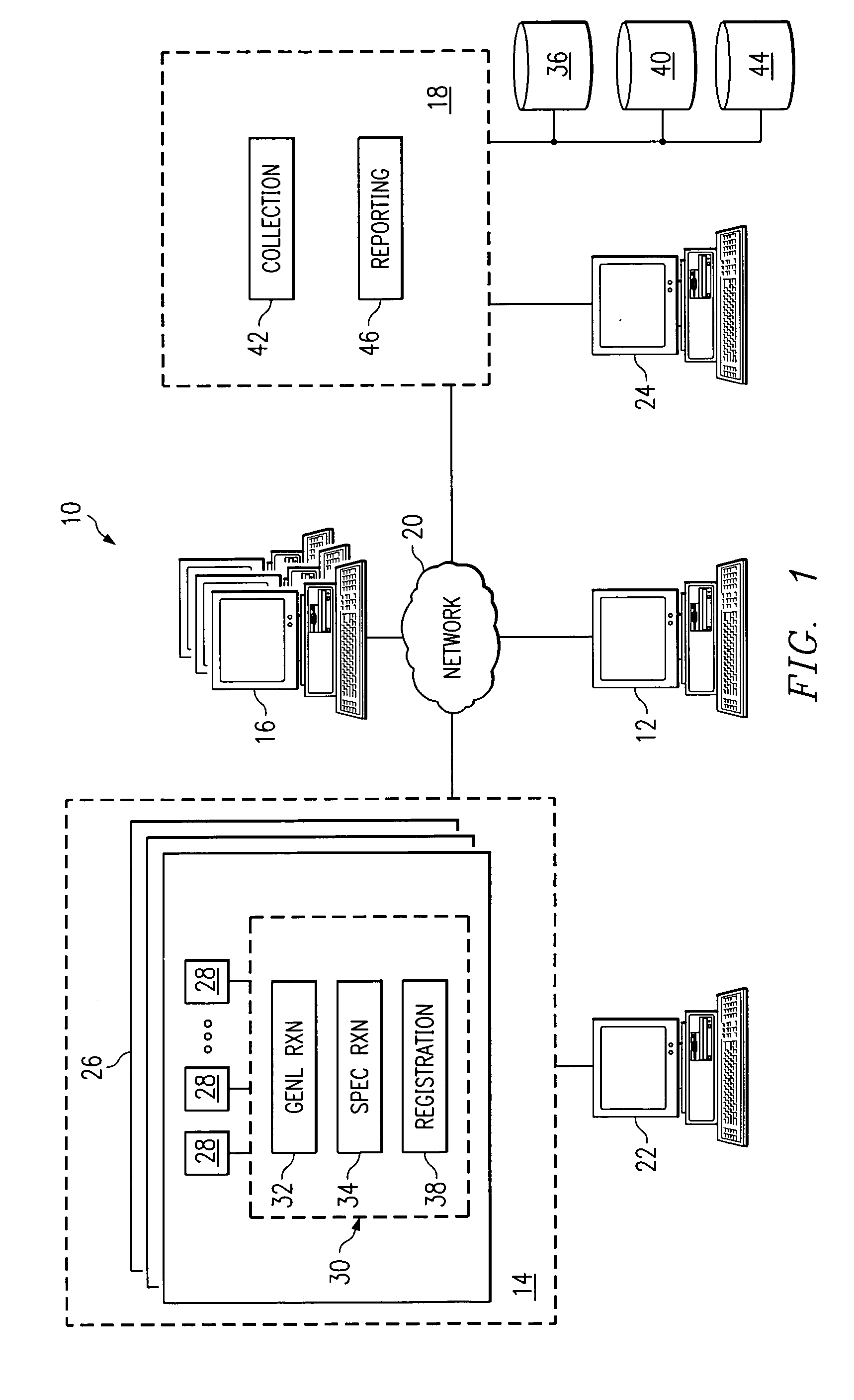
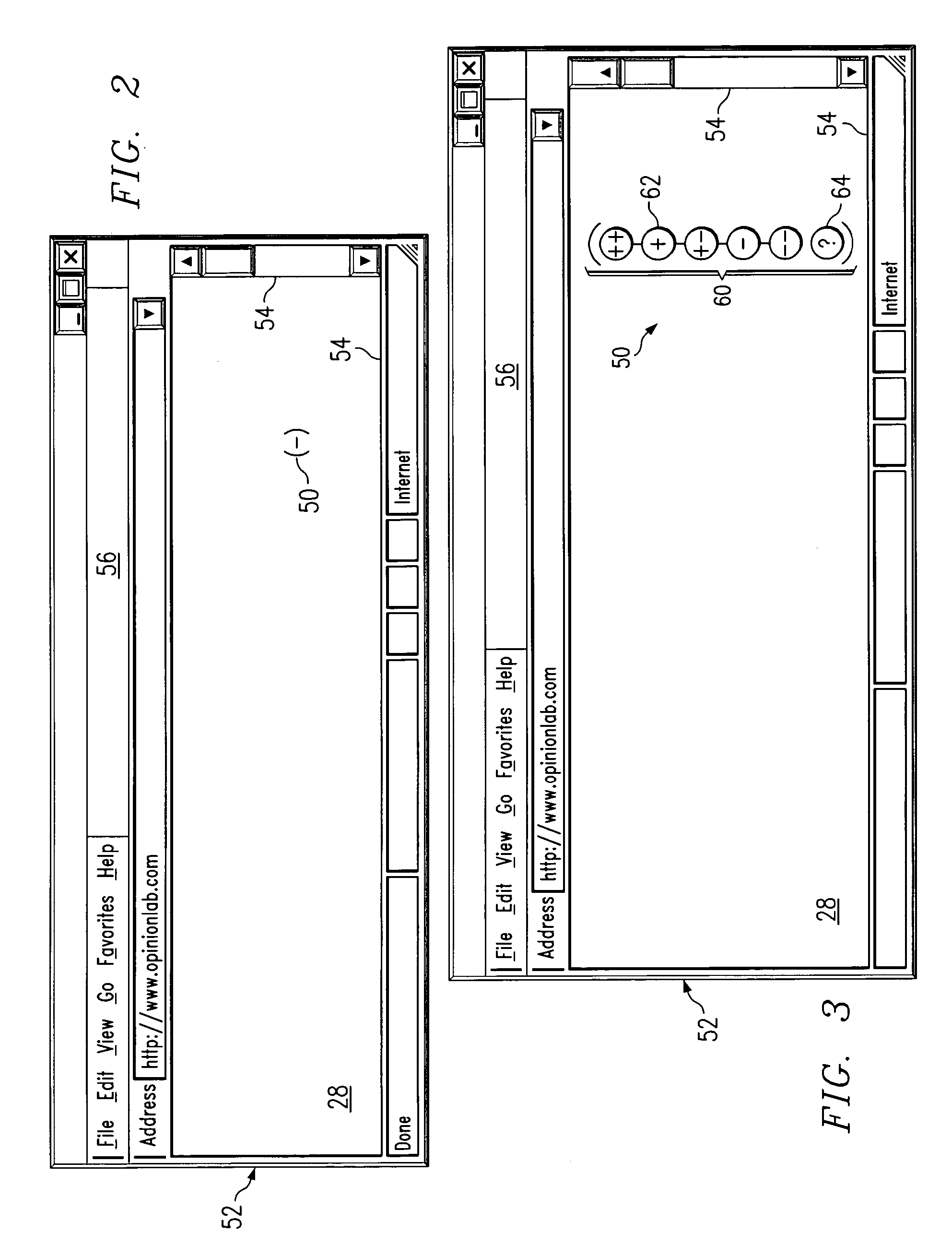
Receiving and reporting page-specific user feedback concerning one or more particular web pages of a website
In one embodiment, a method for receiving page-specific user feedback concerning a particular web page of a website includes using a comment icon viewable on the page to solicit one or more page-specific open-ended comments concerning the page from a user. The method includes using software associated with the comment icon to automatically communicate a request for a comment window to a remote computer system that is separate from a computer system hosting the website in response to the user selecting the comment icon, receive the comment window from the remote computer system, present the comment window to the user, and receive one or more or more page-specific open-ended comments concerning the page from the user provided using the comment window for reporting to a website owner.
Owner:VERINT AMERICAS
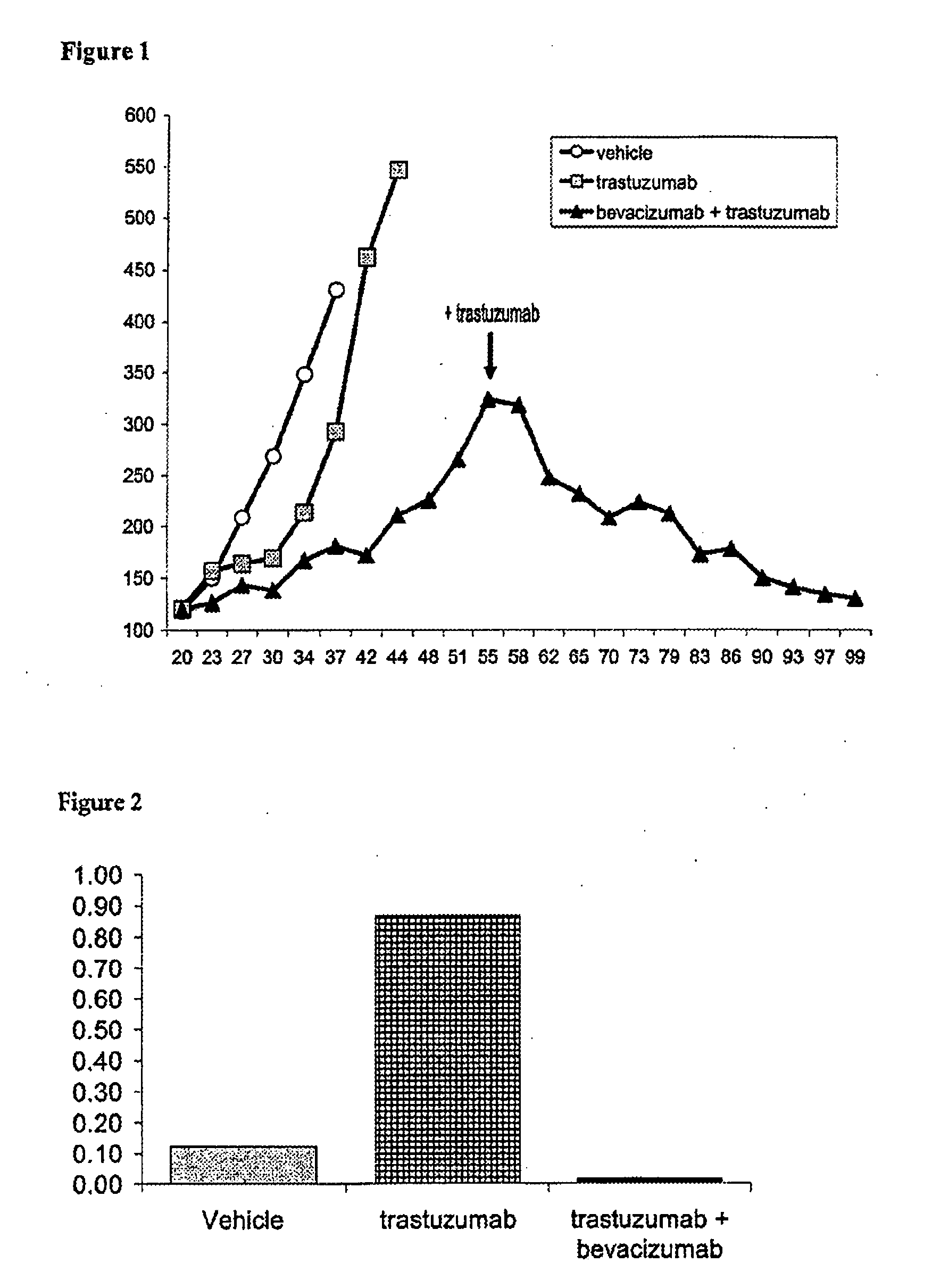
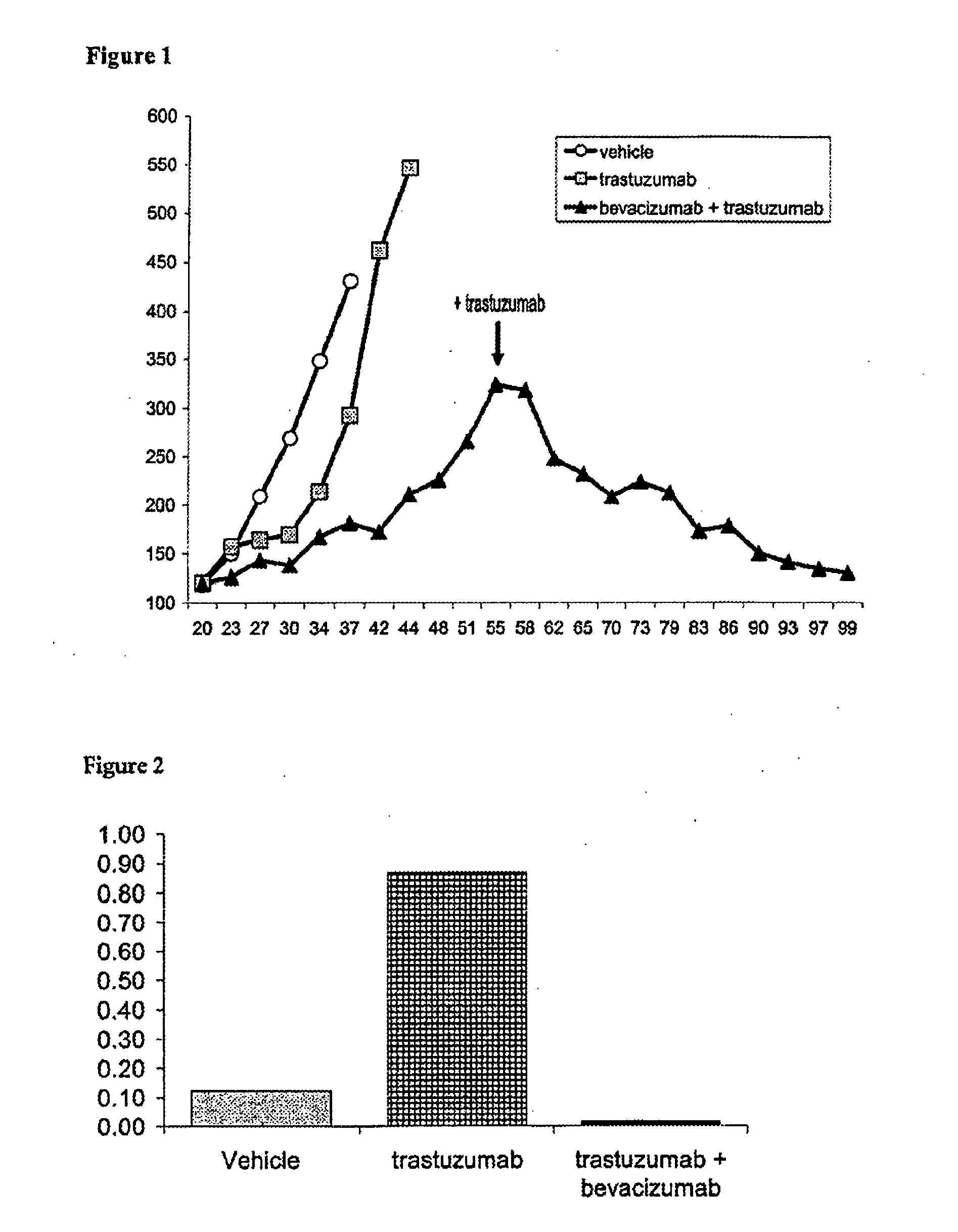
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20070224203A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseTumor therapy
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
Pharmaceutical Combinations of Diazole Derivatives for Cancer Treatment
InactiveUS20090142337A1Low toxicityEliminate side effectsBiocidePeptide/protein ingredientsCancer cellCompound (substance)
The invention provides a combination comprising (or consisting essentially of) an ancillary compound and a compound of the formula (I): or salts, tautomers, solvates and N-oxides thereof; wherein: R1 is 2,6-dichlorophenyl; R2a and R2b are both hydrogen; and R3 is a group: formula (A) where R4 is C1-4 alkyl. The combinations have activity as inhibitors of CDK kinases and inhibit the proliferation of cancer cells.
Owner:ASTEX THERAPEUTICS LTD
Using software incorporated into a web page to collect page-specific user feedback concerning a document embedded in the web page
In one embodiment, a system for measuring subjective user reaction concerning a particular web page comprising an embedded document includes a first icon viewable on the particular web page independent of input from a user subsequent to the user accessing the particular web page. The particular web page includes a document embedded in the particular web page. The first icon solicits a subjective user reaction to the particular web page comprising the document embedded in the particular web page from the user independent of input from the user subsequent to the user accessing the particular web page. The first icon receives user input indicating a desire to provide a subjective user reaction to the particular web page including the document embedded in the particular web page. The user input causes a second icon to become viewable on the particular web page. The second icon provides the user an opportunity to provide a subjective user reaction to the particular web page including the document embedded in the particular web page. The system also includes software associated with the second icon that receives the subjective user reaction to the particular web page including the document embedded in the particular web page for reporting to an owner of the document embedded in the particular web page.
Owner:OPINIONLAB
LED display and manufacturing method thereof
ActiveUS20140312368A1Light weightSmall sizeSolid-state devicesSemiconductor/solid-state device manufacturingLED displayEngineering
A manufacturing method of a LED display is provided. A temporary substrate is provided, wherein the temporary substrate has a first adhesive layer and a plurality of first, second and third LED chips mounted on the first adhesive layer. A first transparent substrate is provided, the transparent substrate has a plurality of pixels disposed thereon, and each of the pixels comprises a first sub-pixel, a second sub-pixel and a third sub-pixel respectively surrounded by a light-insulating structure. Then, the temporary substrate and the first transparent substrate are bonded together, such that each of the first, second and third LED chips is correspondingly mounted in each of the first sub-pixels, the second sub-pixels and the third sub-pixels. After that, the temporary substrate is removed. A LED display manufactured by said method is also provided.
Owner:LEXTAR ELECTRONICS CORP
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
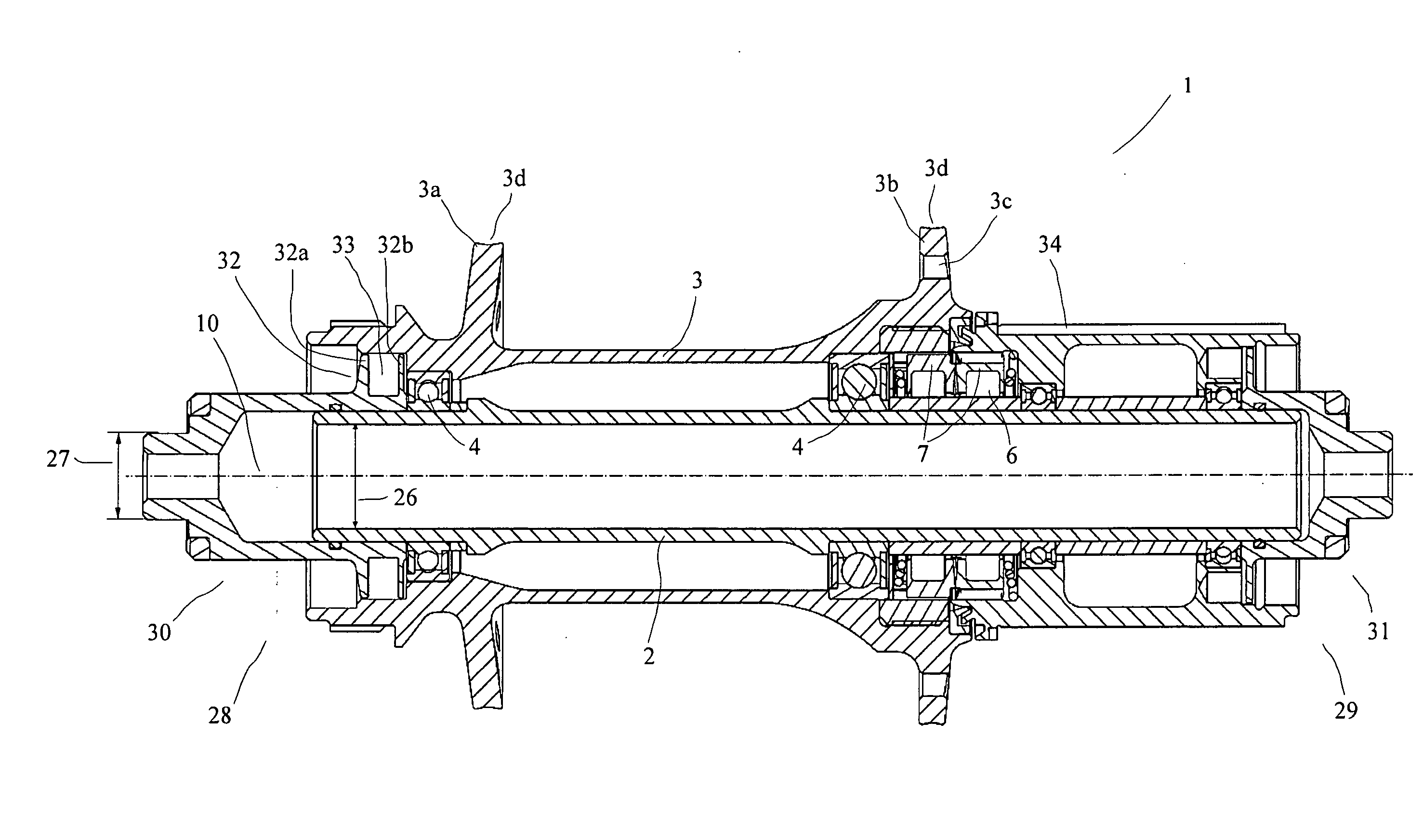
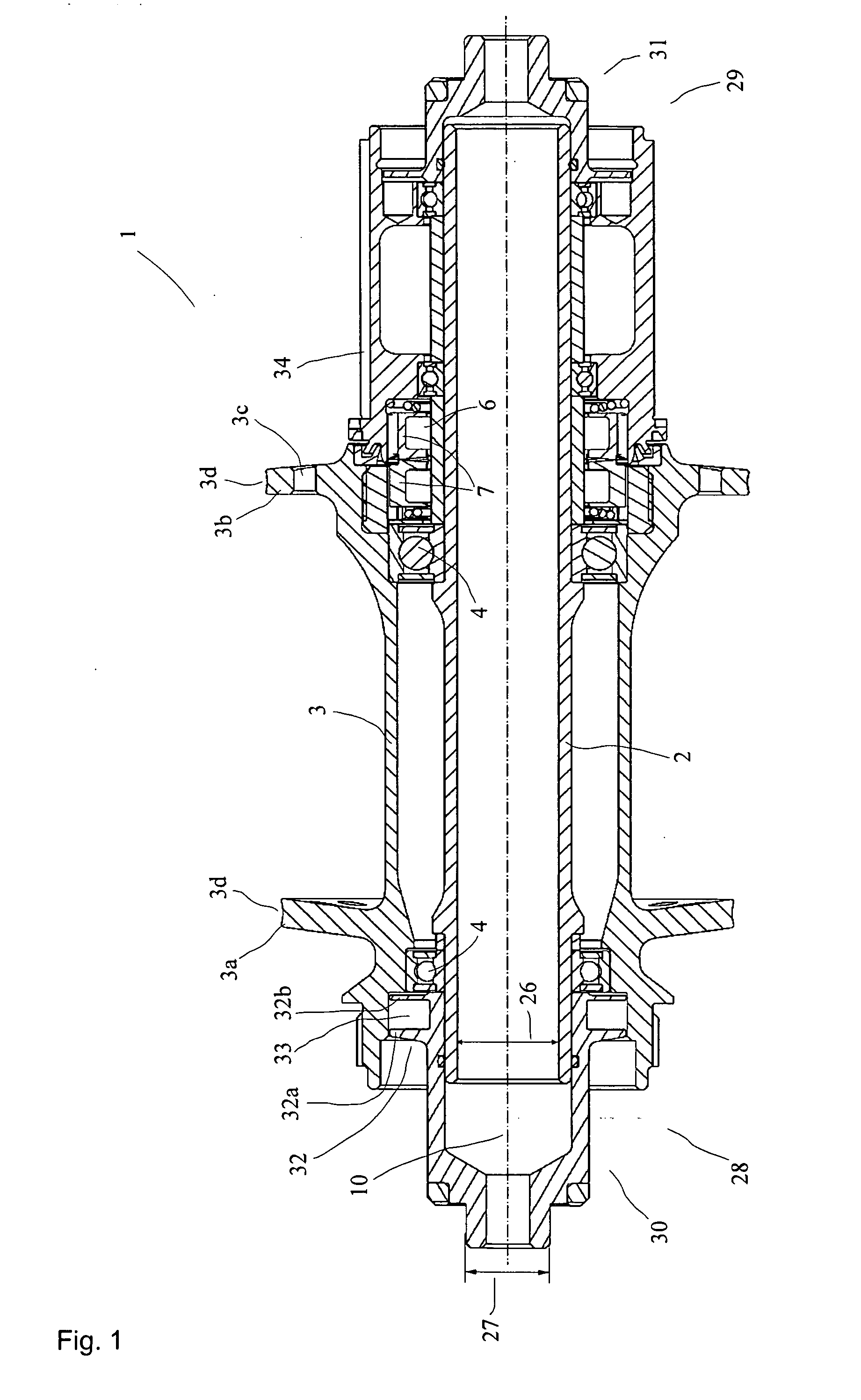
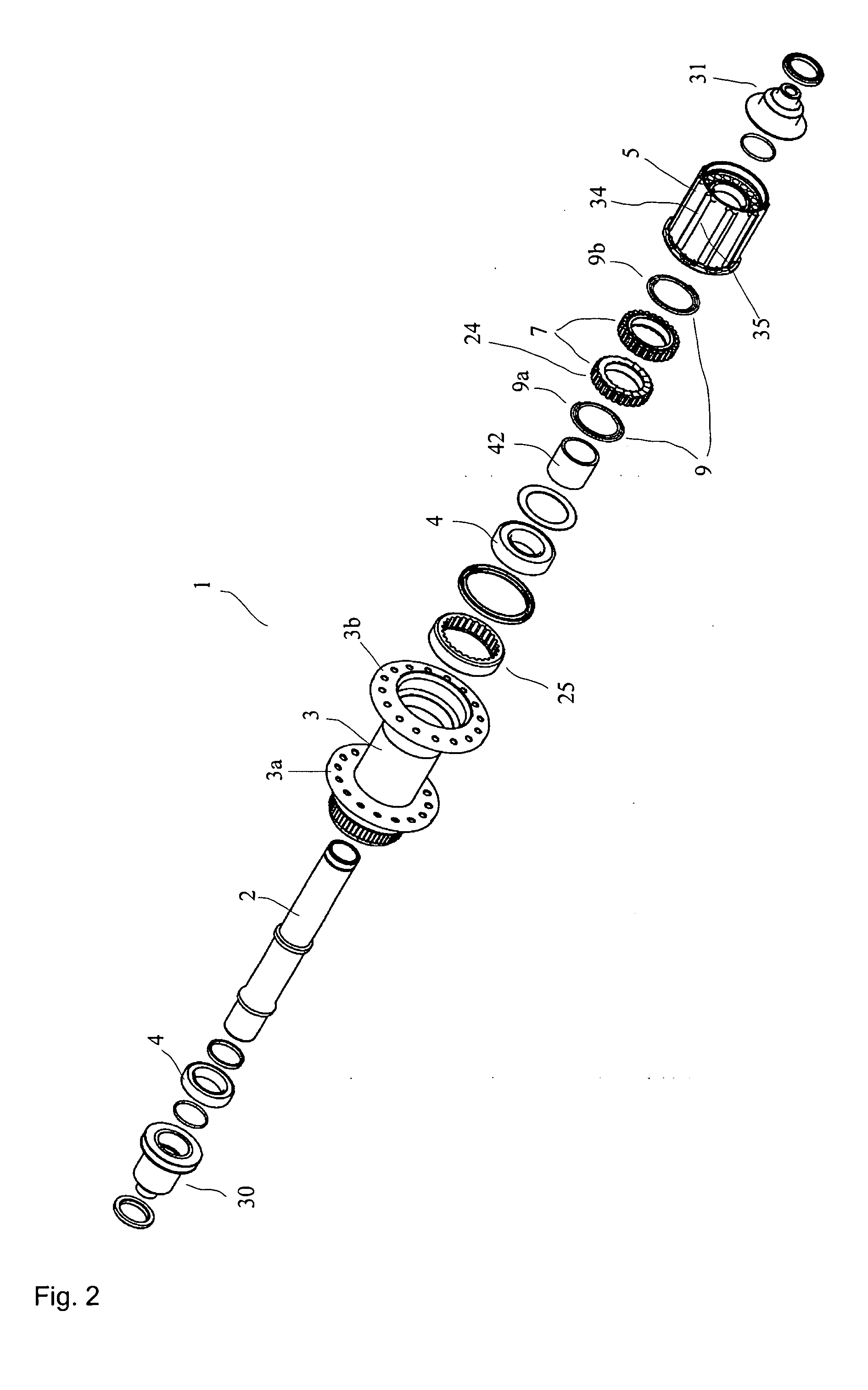
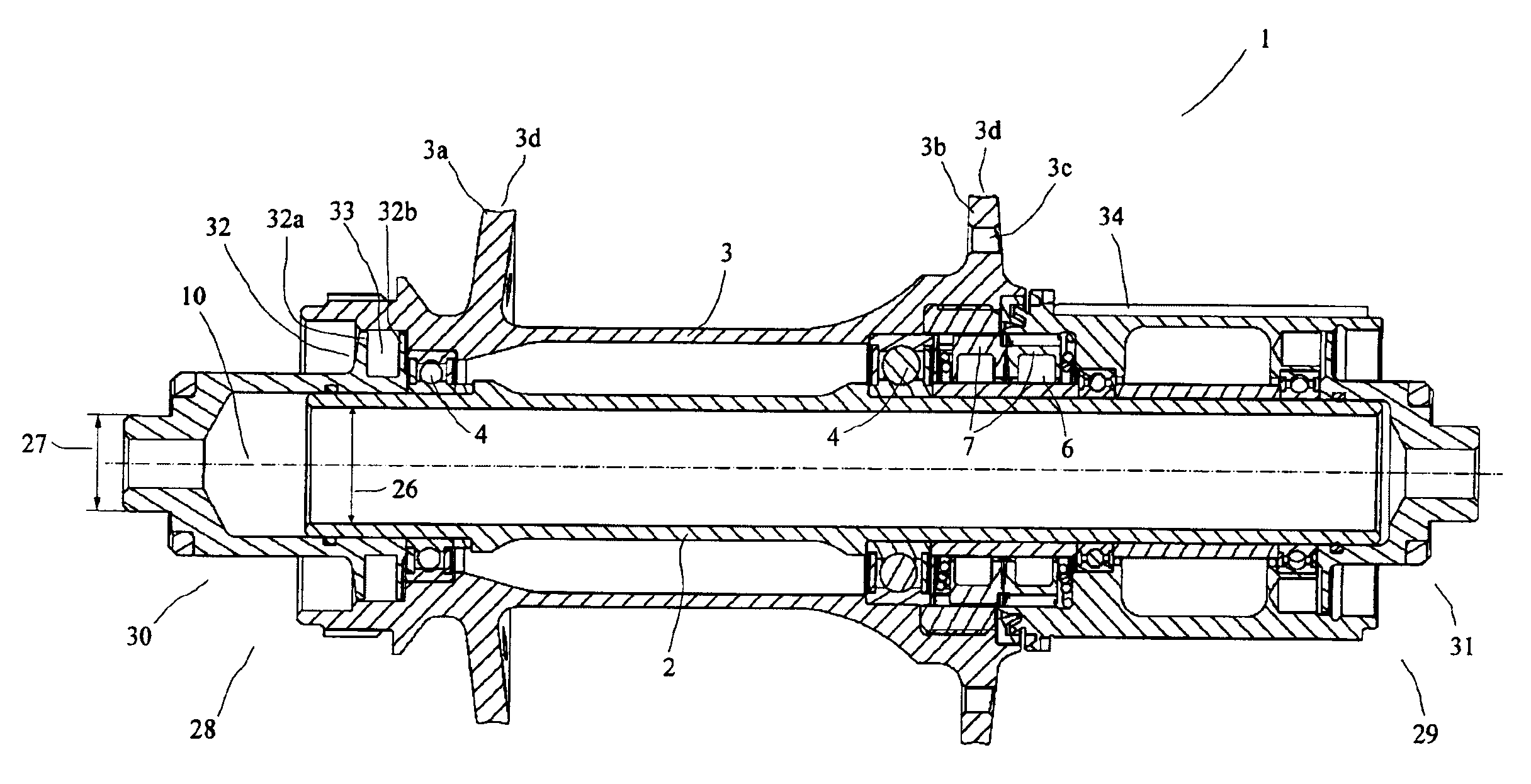
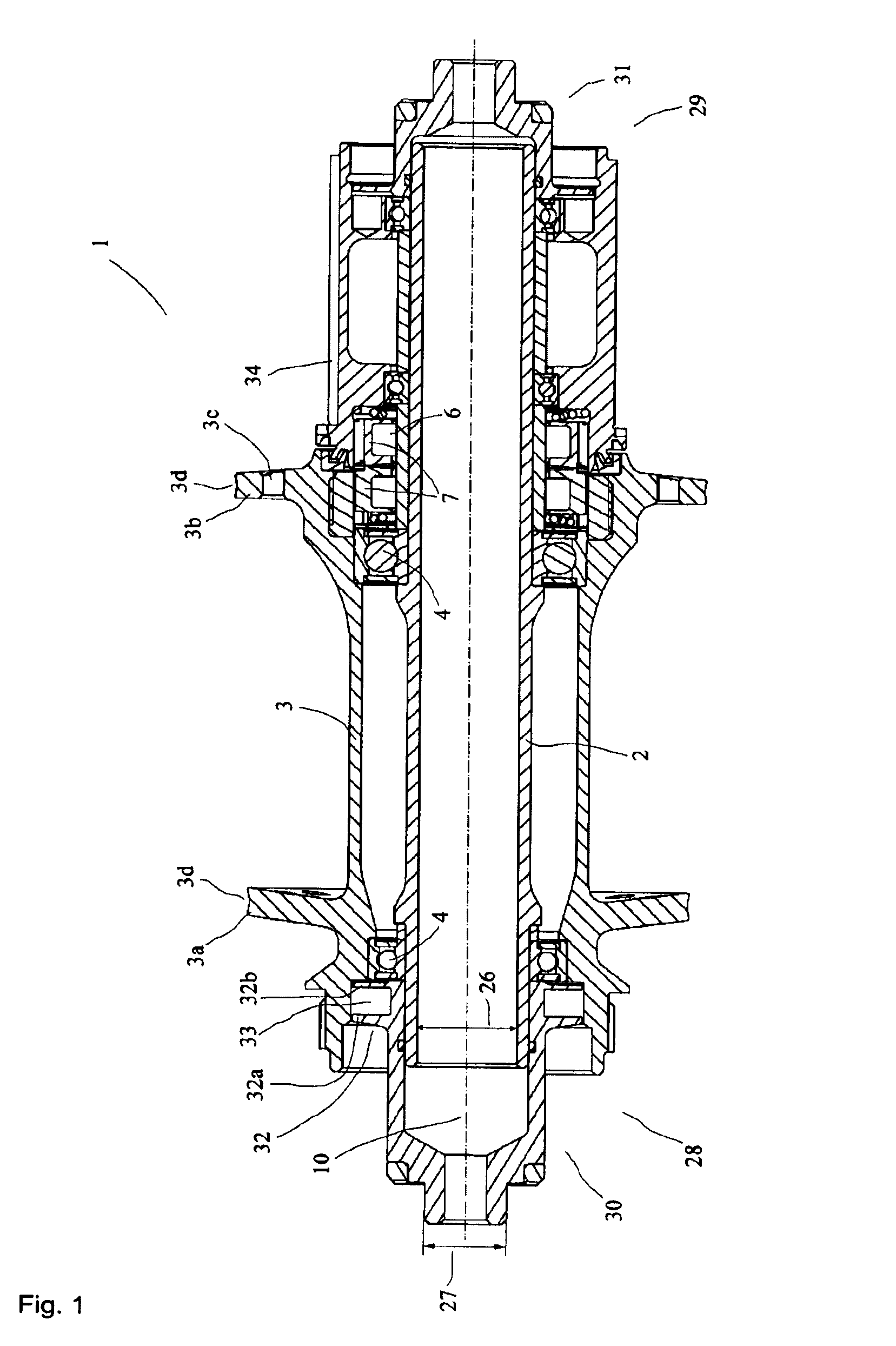
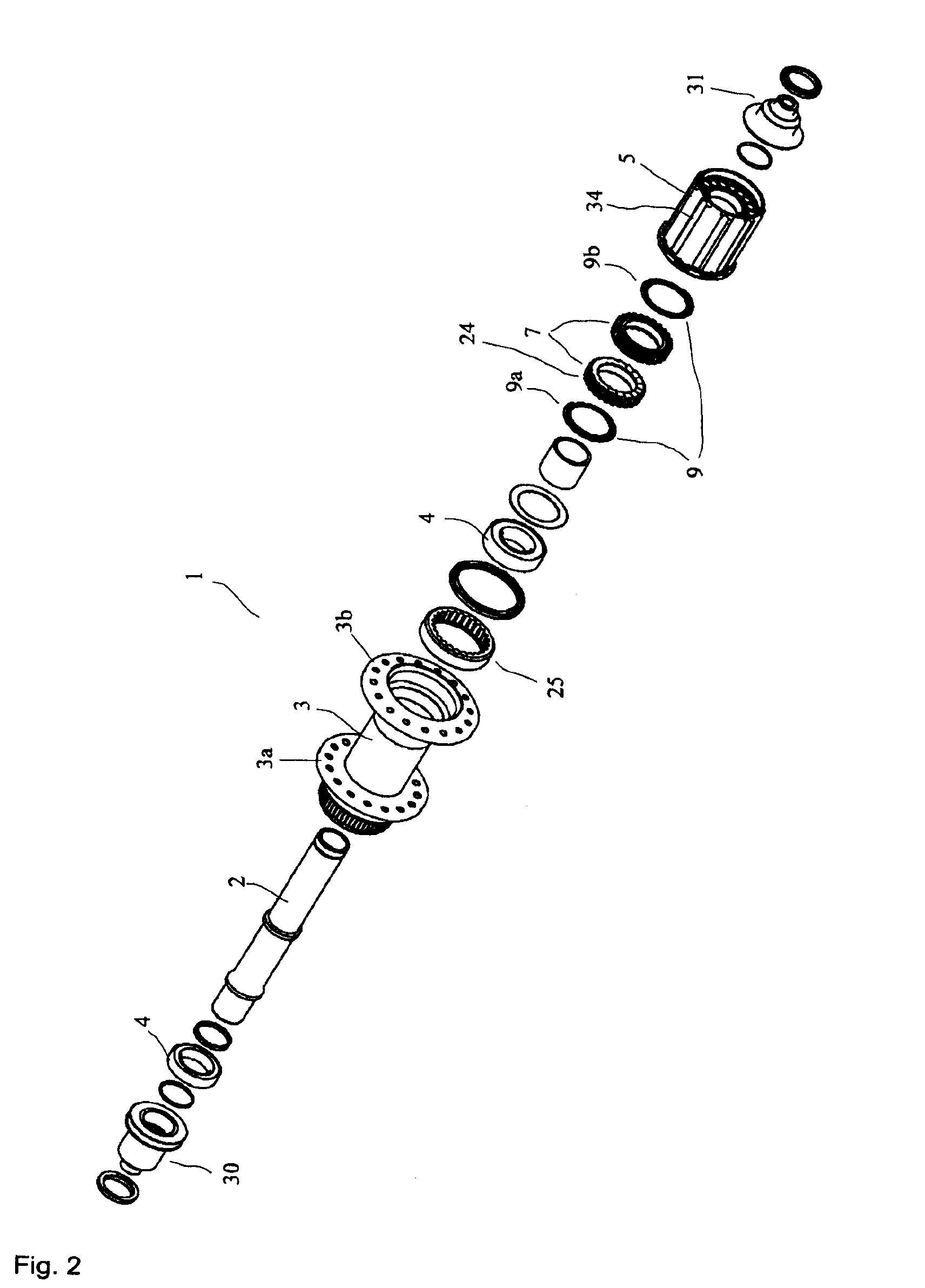
Rear wheel hub, in particular for bicycles
ActiveUS20080006500A1Increased stressabilityMore materialRotating vibration suppressionHubsFreewheelSprocket
A hub for bicycles, having an axle and a shell, two bearing units arranged substantially between the hub axle and the hub shell, and a rotor rotatably supported relative to the hub axle for receiving a sprocket the rotor, and a freewheel having two toothed disc units with biased tooth faces for transmitting the drive torque from the rotor to the hub shell. The two toothed disc units are disposed around the hub axle upon assembly, they are, at idle, disposed transverse to the hub axle. At least one toothed disc unit includes a toothed disc having a hole, a radial wall and an axial toothed wall, wherein an inner diameter of the hole is smaller at the axial wall than an inner dimension in the radial wall to form a receiving space at the toothed disc between the clear inner diameter of the axial wall and the radial wall.
Owner:SWISS
Electrochromic or electrodeposition display and novel process for their manufacture
ActiveUS7245414B2Extend your lifeImprove throughputNon-linear opticsOptical elementsEngineeringElectrochromism
The present invention is directed to an improved electrodeposition and an electrochromic display. The display cells formed from microcups are filled with an electrolyte fluid and are individually sealed with a polymeric sealing layer.
Owner:E INK CORPORATION
Method and system of providing a search tool
InactiveUS20150178392A1High response rateDigital data information retrievalDigital data processing detailsMachine learning
A method and system of improving user experience in a human assisted search system is described. The disclosed method and system allows a search system to provide a personalized response to a user by utilizing interconnecting characteristics determined by the search system to be used. A search request or “query” may be processed automatically and / or utilizing the assistance of a human assistant.
Owner:CHACHA SEARCH
Methods of determining acute myeloid leukemia response to treatment with farnesyltransferase
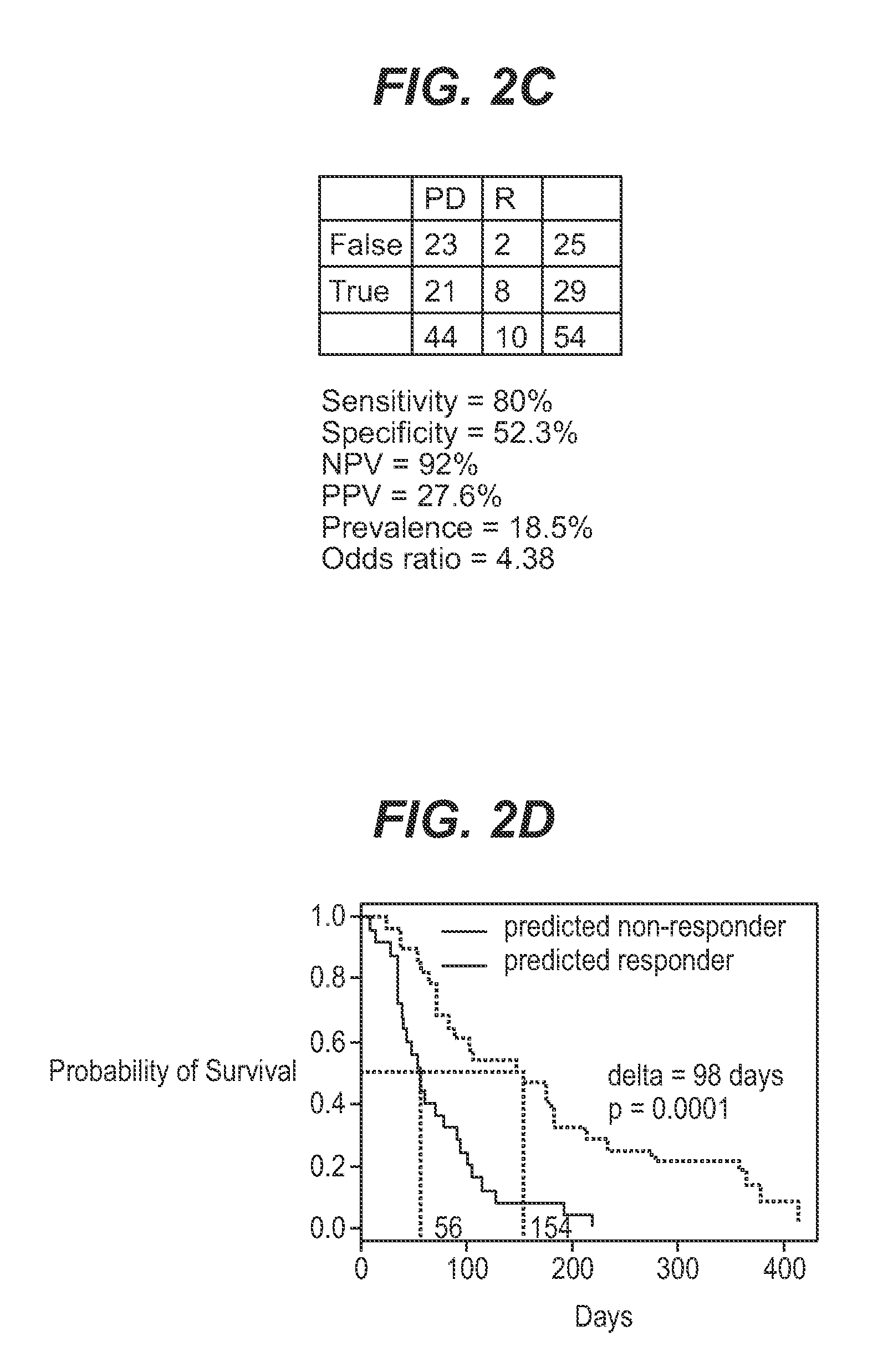
InactiveUS7932036B1Improve accuracyHigh response rateSugar derivativesMicrobiological testing/measurementNewly diagnosedClinical study
We analyzed bone marrow from 67 patients from a phase 2 study of farnesyltransferase inhibition with tipifarnib (R115777, ZARNESTRA®), in older adults with previously untreated, poor-risk acute myeloid leukemia (AML) for N-Ras mutations, global gene expression, and / or quantitative PCR (qPCR) of specific genes. Microarray profiling identified a two-gene expression ratio (RASGRP1:APTX) which provided the greatest accuracy for predicting response to tipifarnib. We demonstrated that this classifier could predict response to tipifarnib in an independent set of 54 samples from relapsed or refractory AML, with a NPV and PPV of 92% and 28%, respectively (odds ratio of 4.4). Therefore, in both newly diagnosed and relapsed or refractory AML, this classifier improves the overall response rate by approximately 50% while maintaining a high NPV, and significantly improves patient overall survival. The two-gene classifier was also validated by qPCR in thirty AML samples from the same clinical study demonstrating a negative predictive value (NPV) and positive predictive value (PPV) of 81% and 50%, respectively (odds ratio of 4.3). These data indicate that a simple two-gene expression assay may have utility in diagnosing a population of AML patients who are more likely to respond to tipifarnib.
Owner:JANSSEN DIAGNOSTICS LLC
Vehicle behavior control system
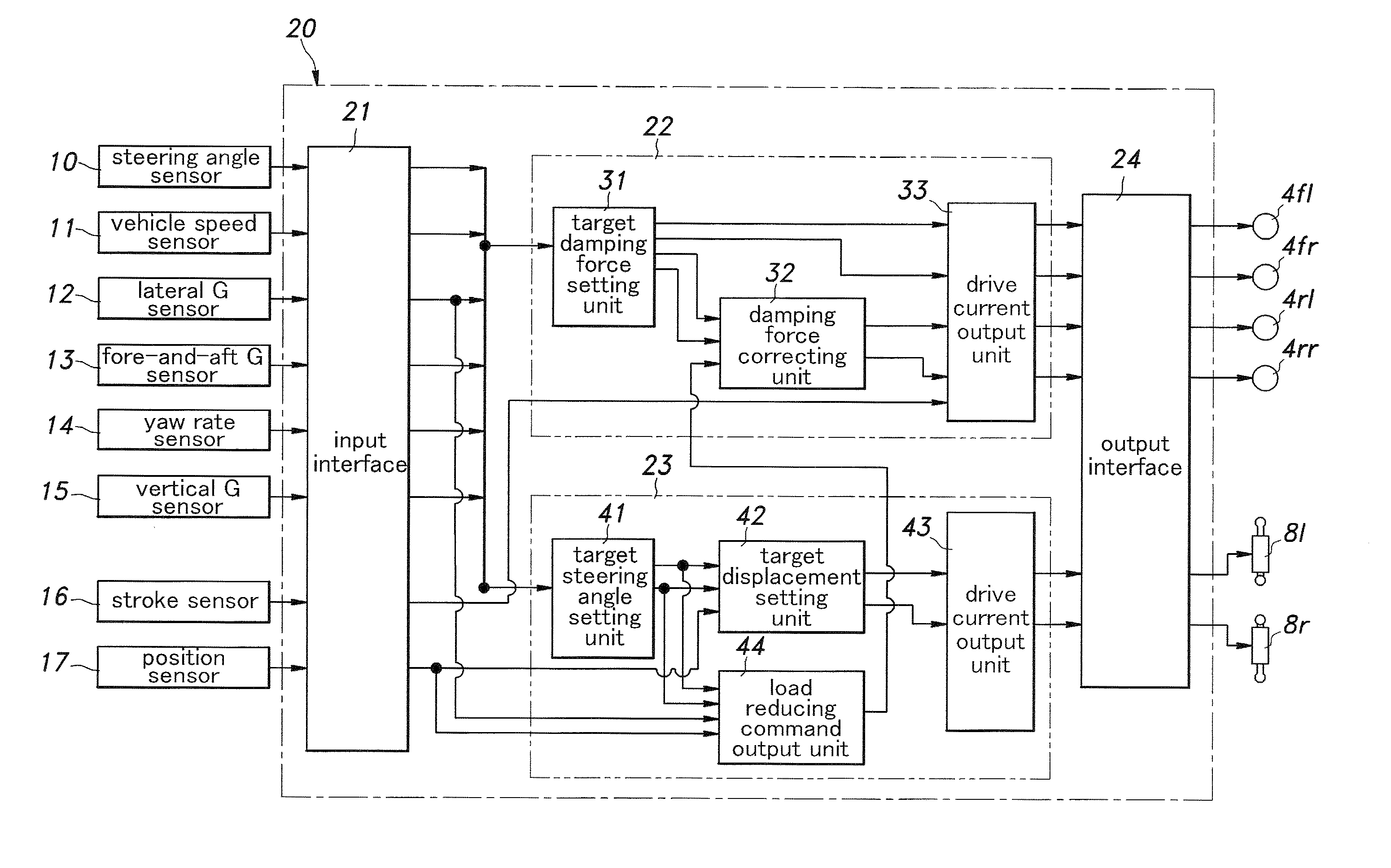
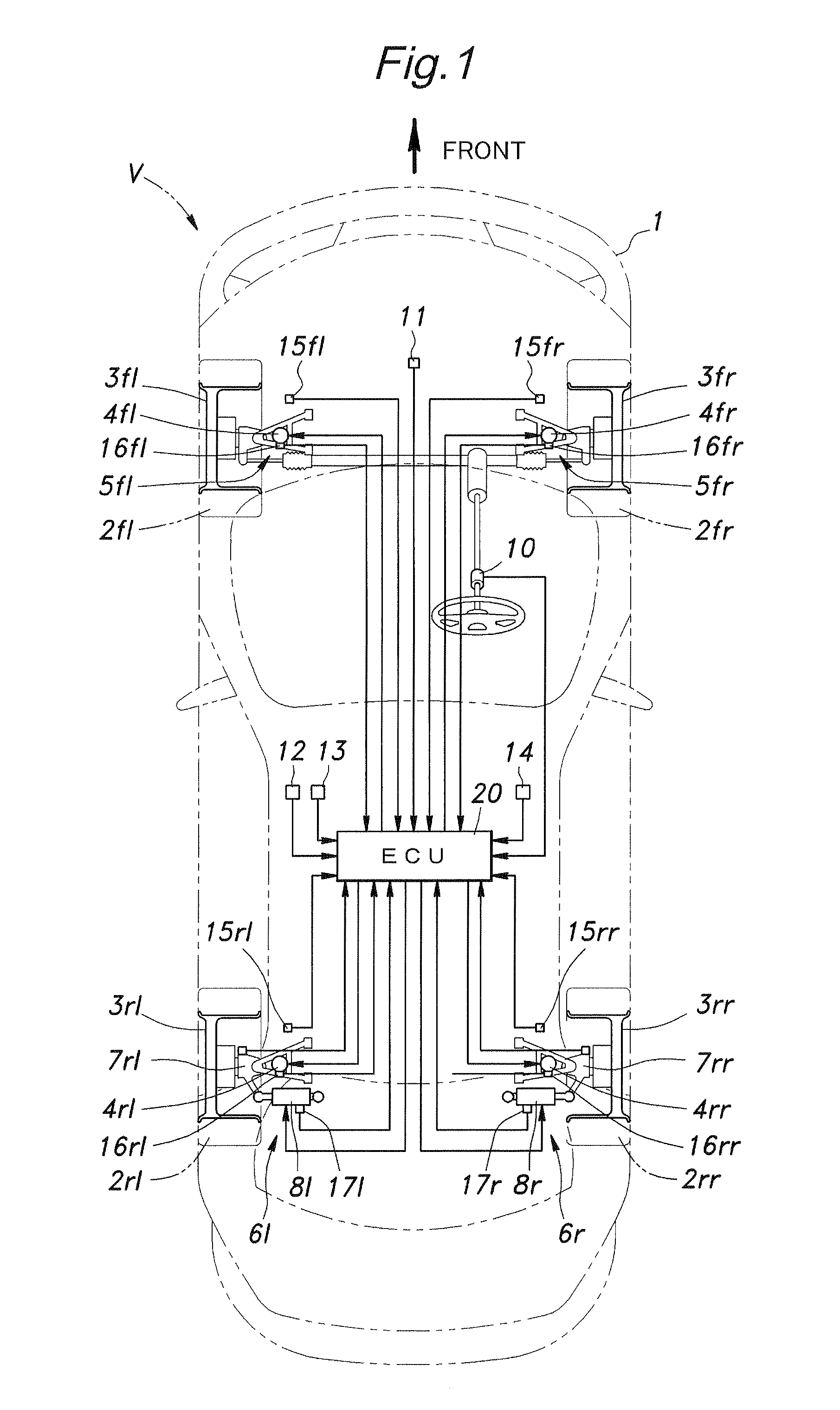
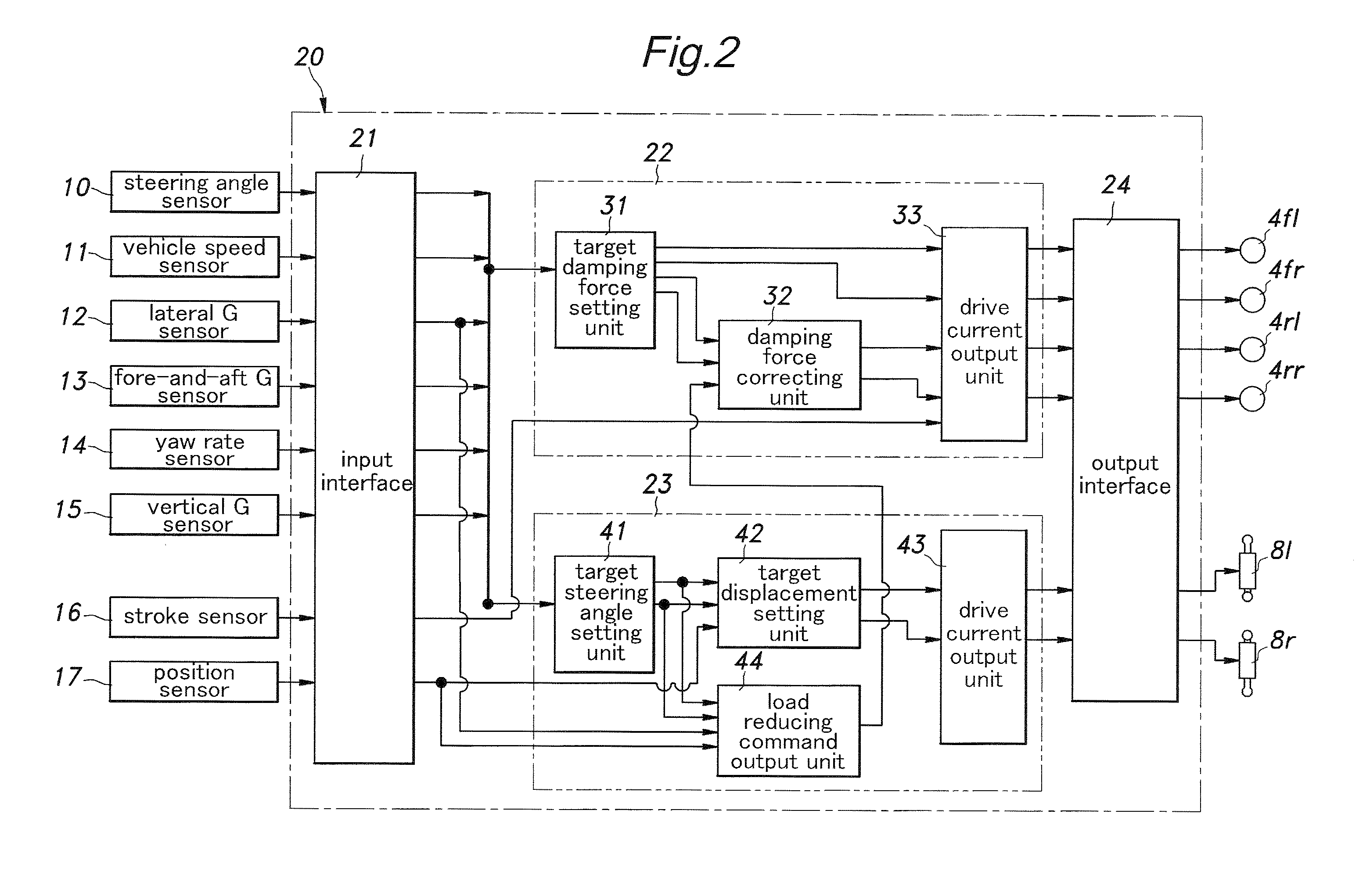
InactiveUS20100211261A1High response rateReduced torque outputDigital data processing detailsAnimal undercarriagesBehavior controlVehicle behavior
Provide is a vehicle behavior control system that can achieve a rear wheel steering control demonstrating a high rate of response at all times without requiring the actuator for the rear wheels to be steered to be unduly increased. A control unit (20) for controlling the rear wheel steering angle is configured to reduce the effective stiffness of the rear wheel suspension systems while increasing the effective stiffness of the front wheel suspension systems when an increase in the road contact load of one of the rear wheels is detected or estimated. Thereby, an actuator (8) for the rear wheel is enabled to steering the rear wheels without involving any undue time delay with a minimum power requirement.
Owner:HONDA MOTOR CO LTD
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20110064736A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseFactor ii
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
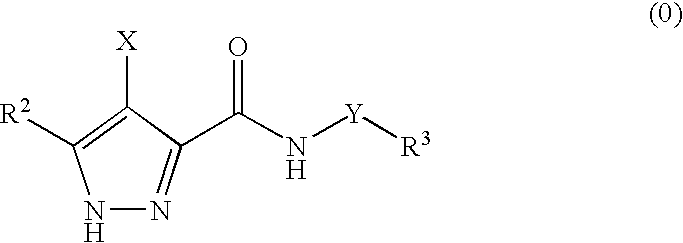
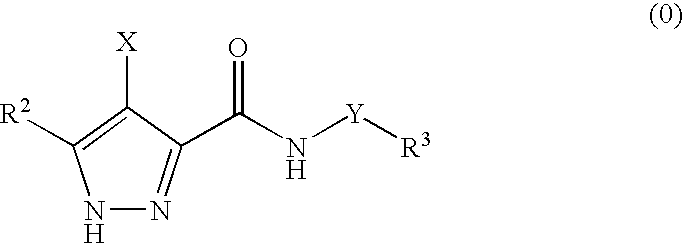
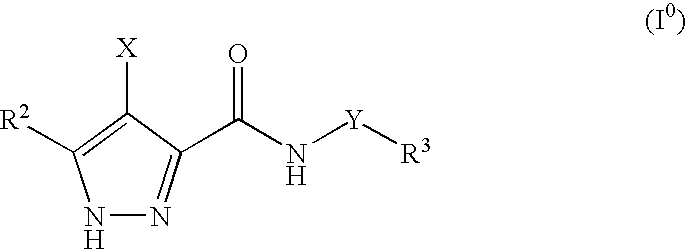
Pharmaceutical combinations
The invention provides a combination comprising an ancillary compound and a compound having the formula (0): or salts or tautomers or N-oxides or solvates thereof; wherein X is a group R1-A-NR4— or a 5- or 6-membered carbocyclic or heterocyclic ring; A is a bond, SO2, C═O, NR9(C═O) or 0(C═O) wherein R9 is hydrogen or C1-4 hydrocarbyl optionally substituted by hydroxy or C1-4 alkoxy; Y is a bond or an alkylene chain of 1, 2 or 3 carbon atoms in length; R1 is hydrogen; a carbocyclic or heterocyclic group having from 3 to 12 ring members; or a C1-8 hydrocarbyl group optionally substituted by one or more substituents selected from halogen, hydroxy, C1-4 hydrocarbyloxy, amino, mono- or di-C1-4 hydrocarbylamino, and carbocyclic or heterocyclic groups having from 3 to 12 ring members, and wherein 1 or 2 of the carbon atoms of the hydrocarbyl group may optionally be replaced by an atom or group selected from O, S, NH, SO, SO2; R2 is hydrogen; halogen; C1-4 alkoxy; or a C1-4 hydrocarbyl group optionally substituted by halogen, hydroxyl or C1-4 alkoxy; R3 is selected from hydrogen and carbocyclic and heterocyclic groups having from 3 to 12 ring members; and R4 is hydrogen or a C1-4 hydrocarbyl group optionally substituted by halogen, hydroxyl or C1-4 alkoxy.
Owner:ASTEX THERAPEUTICS LTD
Liquid crystal display device
InactiveUS20050078081A1Eliminate “ ghost ”Improve display qualityTelevision system detailsStatic indicating devicesStable stateLiquid-crystal display
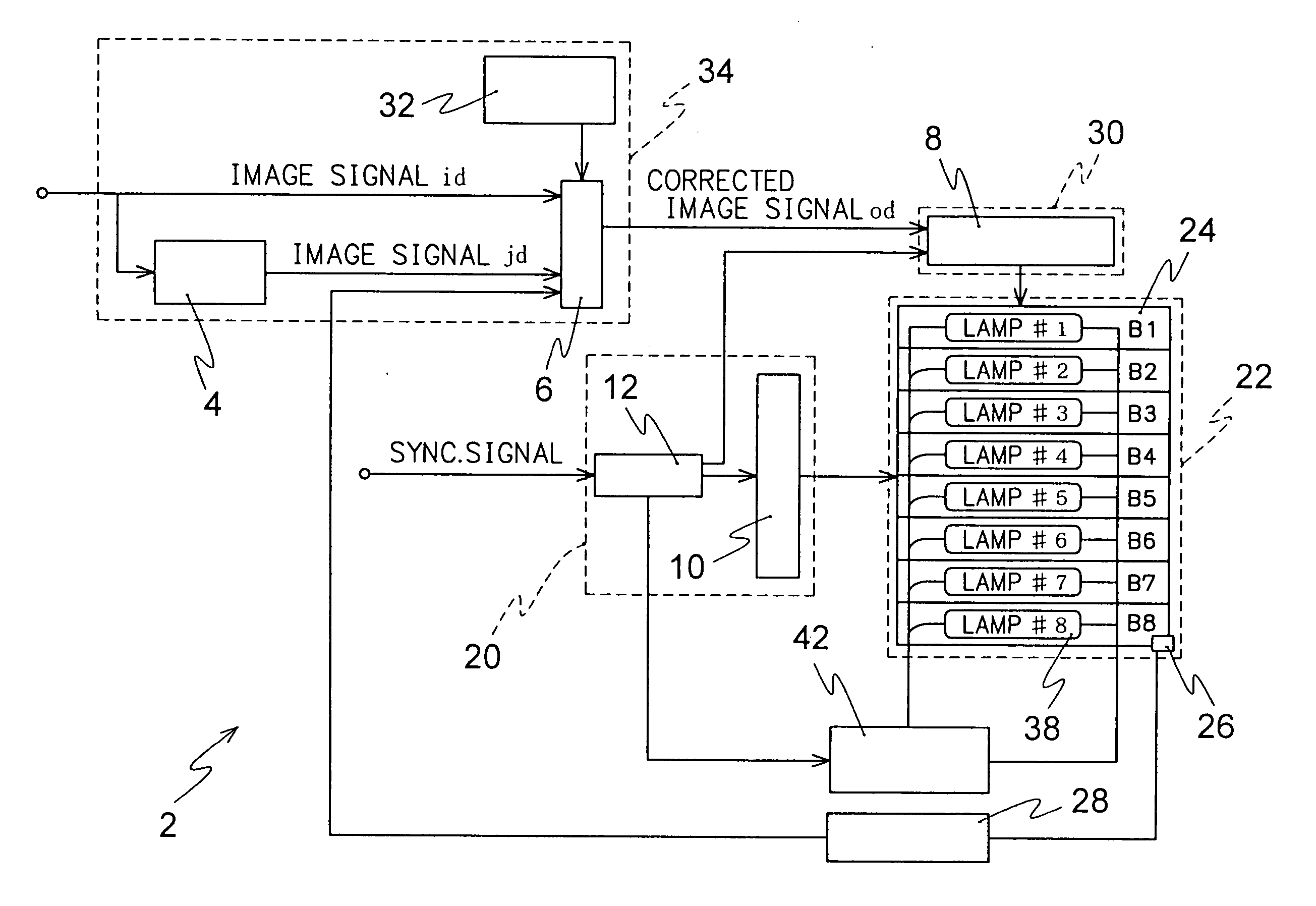
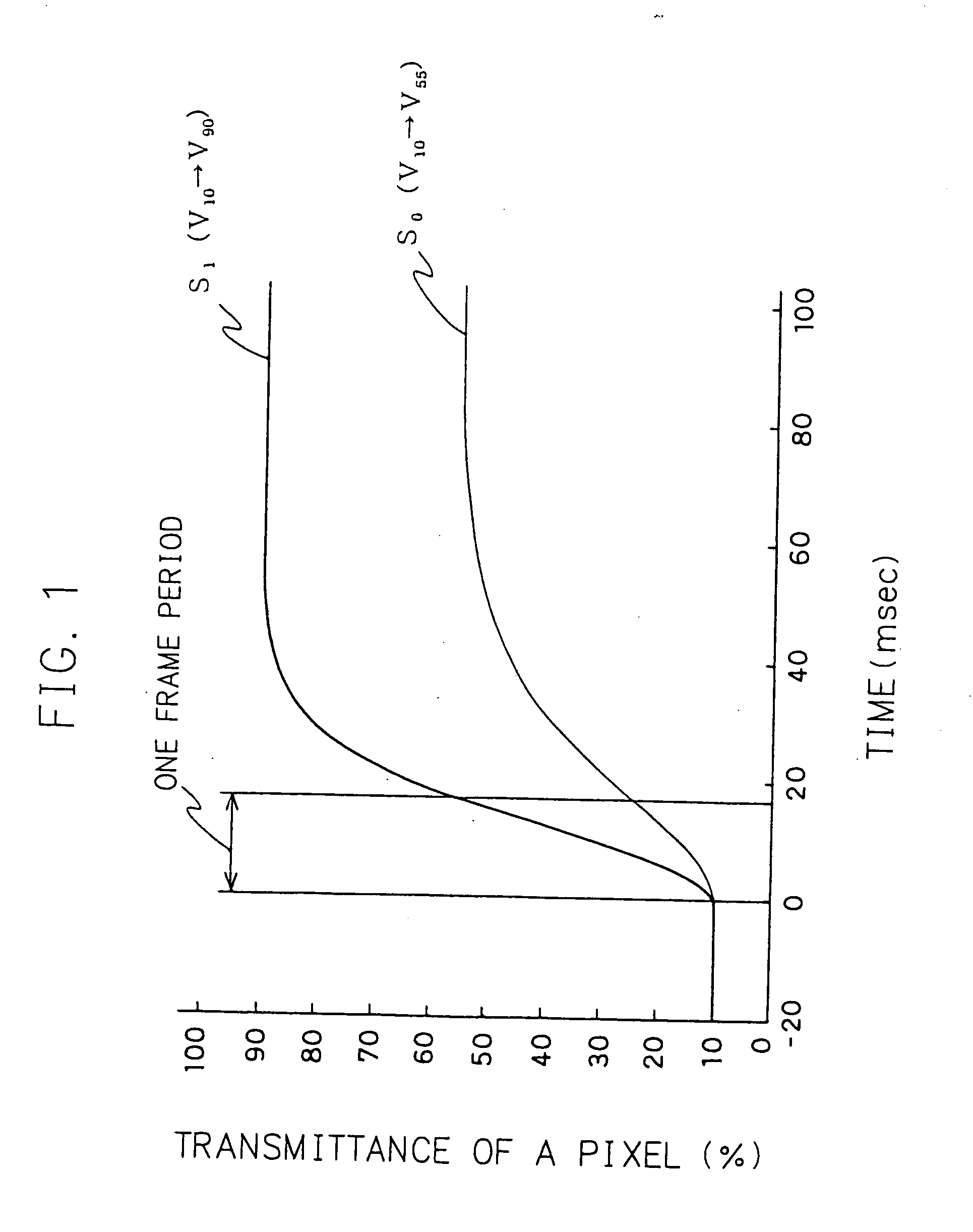
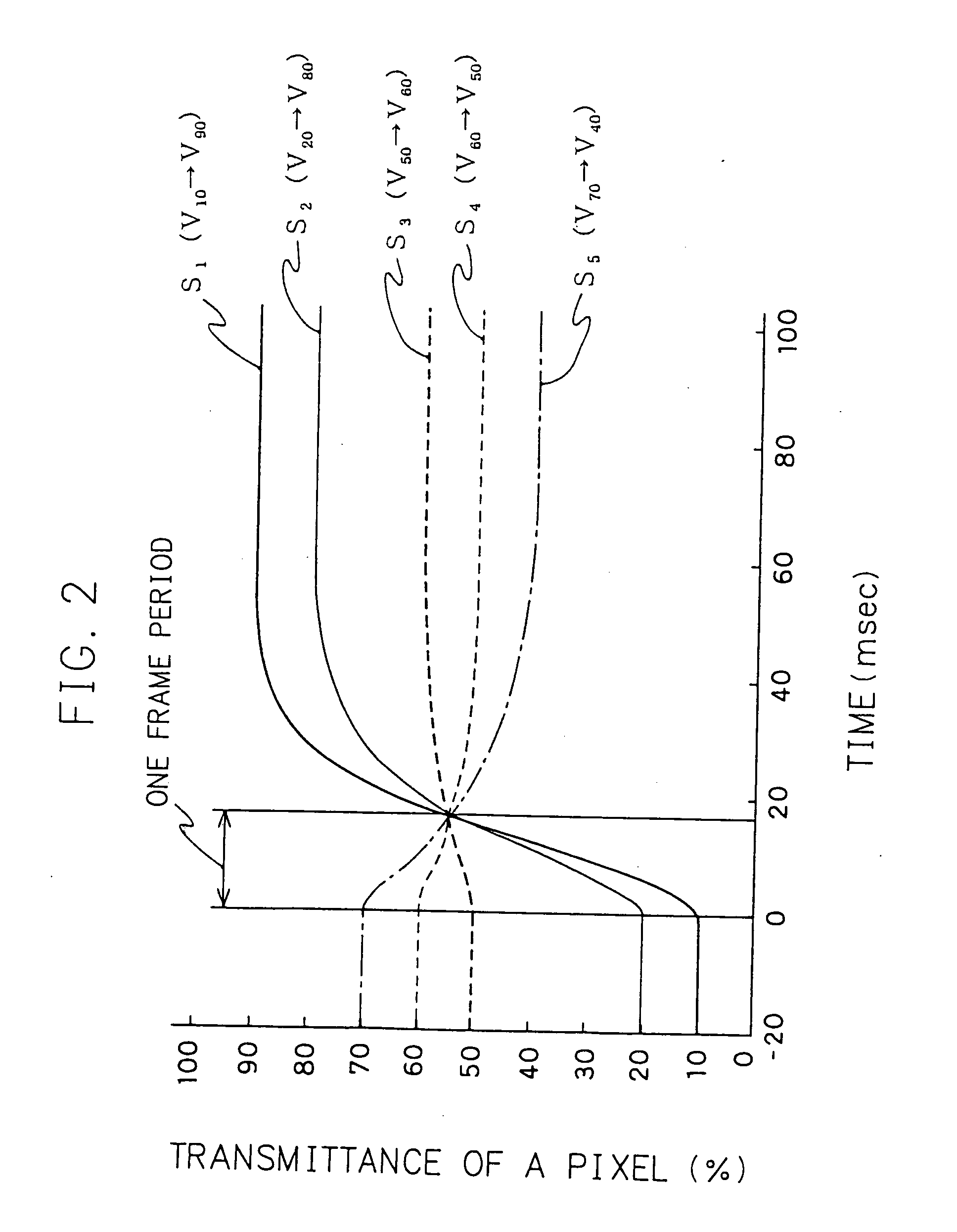
A liquid crystal display device comprising a including signal correcting means correction for correcting a level of an original image signal to a level with which transmittance in a steady state of the pixel with the original image signal is attained within one frame period, a horizontal driving means for driver applying a voltage in correspondence with the corrected image signal to a liquid crystal material, and an illumination device for illuminating the display panel with a plurality of light emitting regions thereof, said the light emitting regions sequentially turns turning on and off in synchronization with the application of the corrected image signal, while holding a definite time delay thereto.
Owner:MITSUBISHI ELECTRIC CORP +1
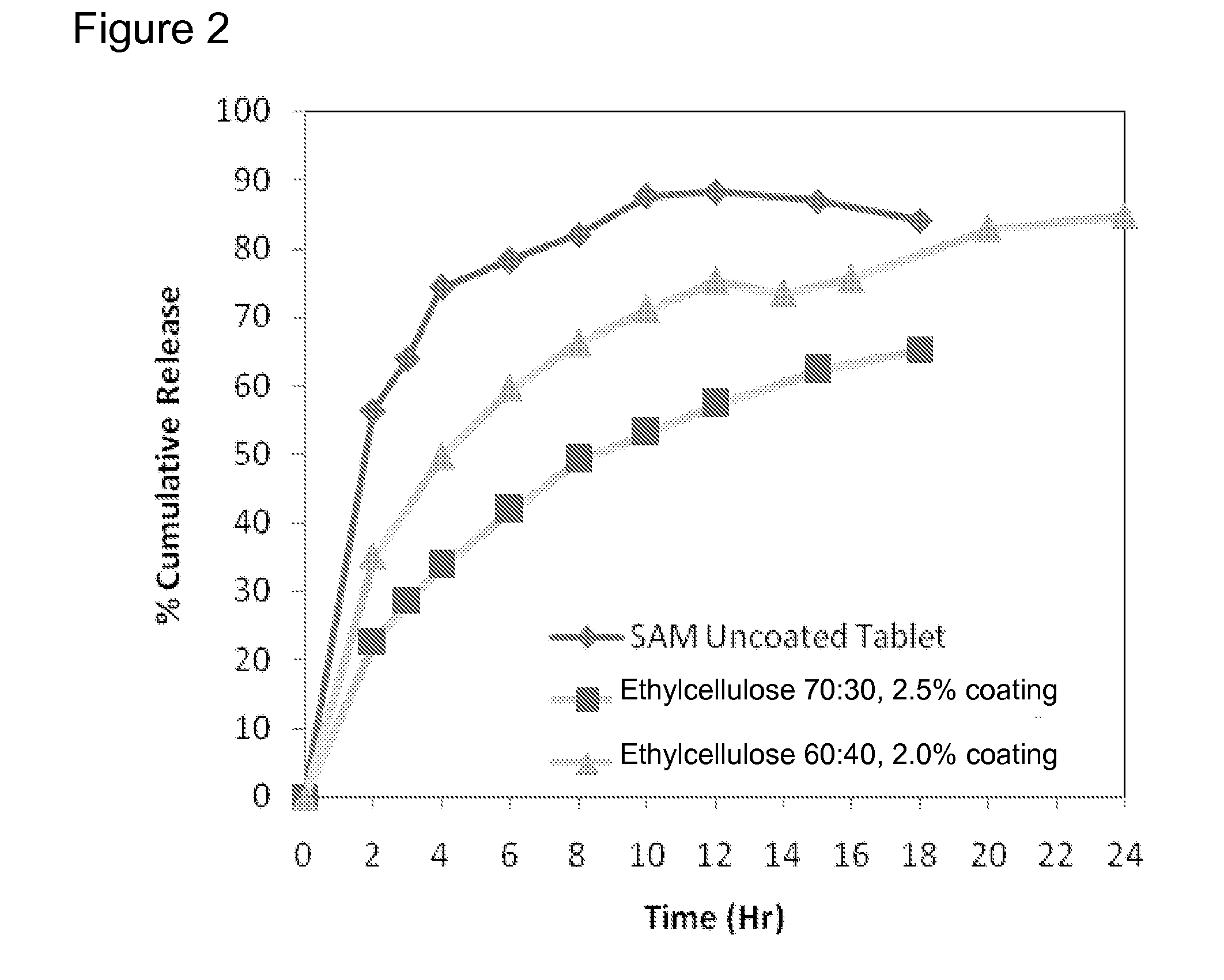
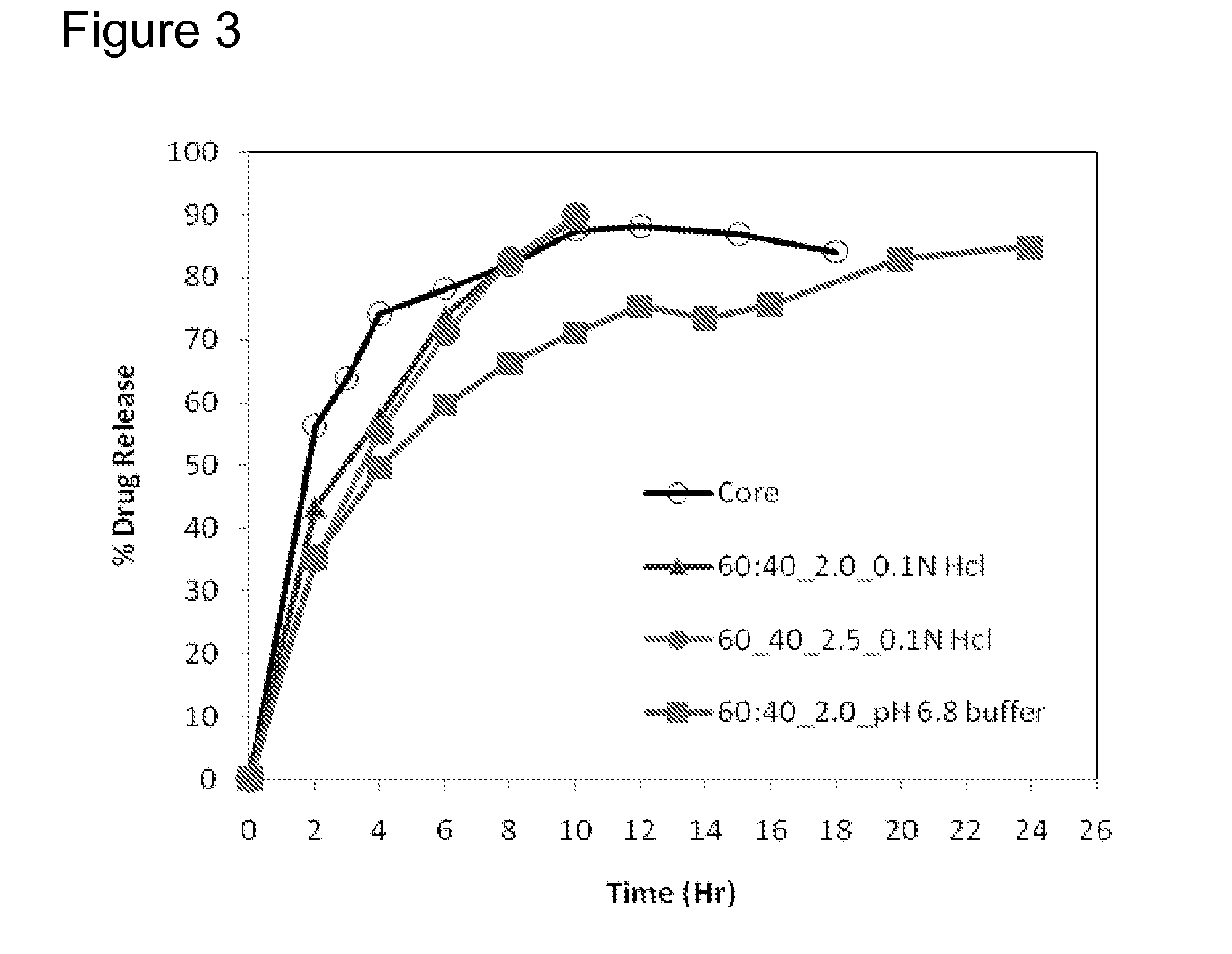
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
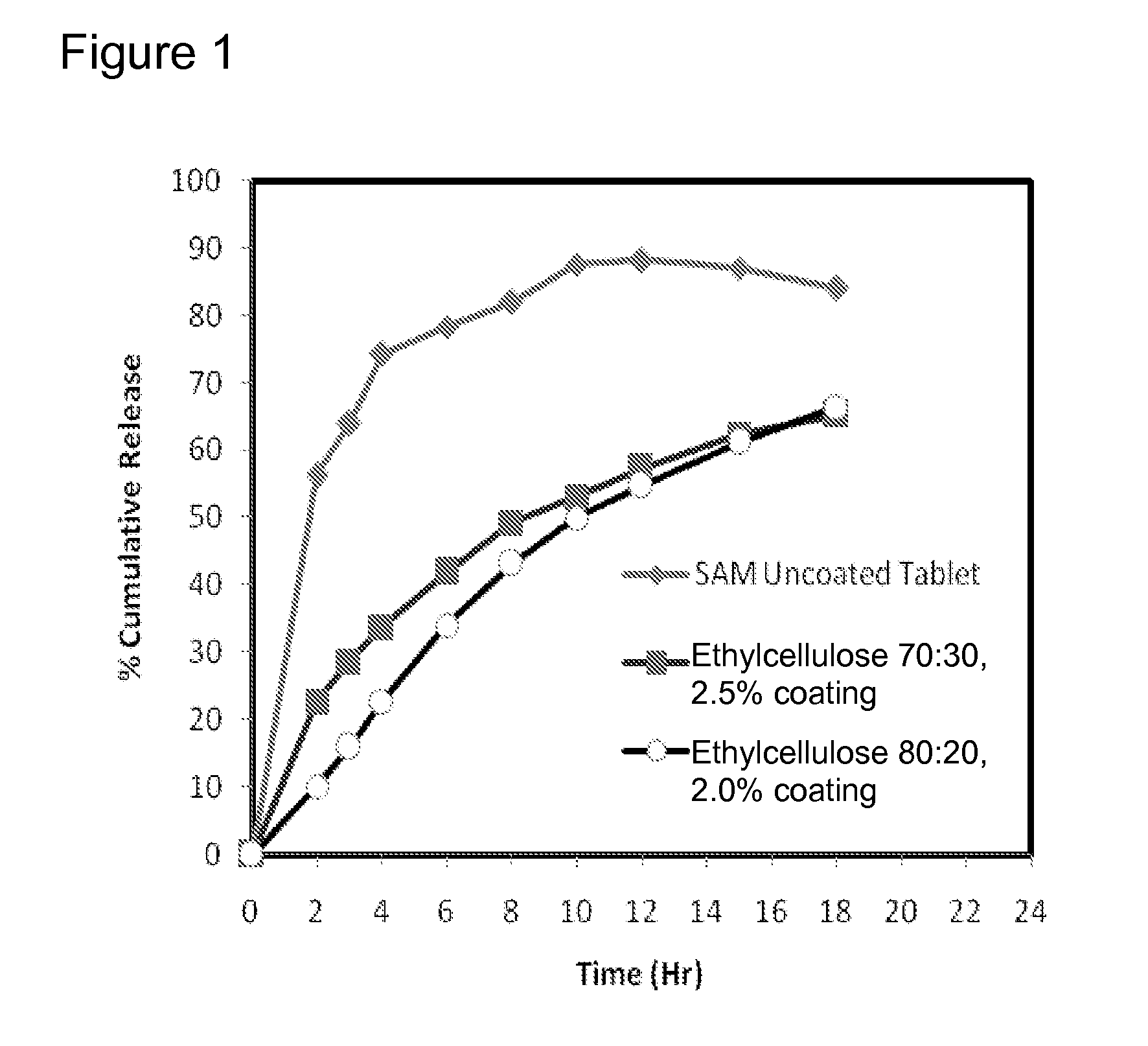
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
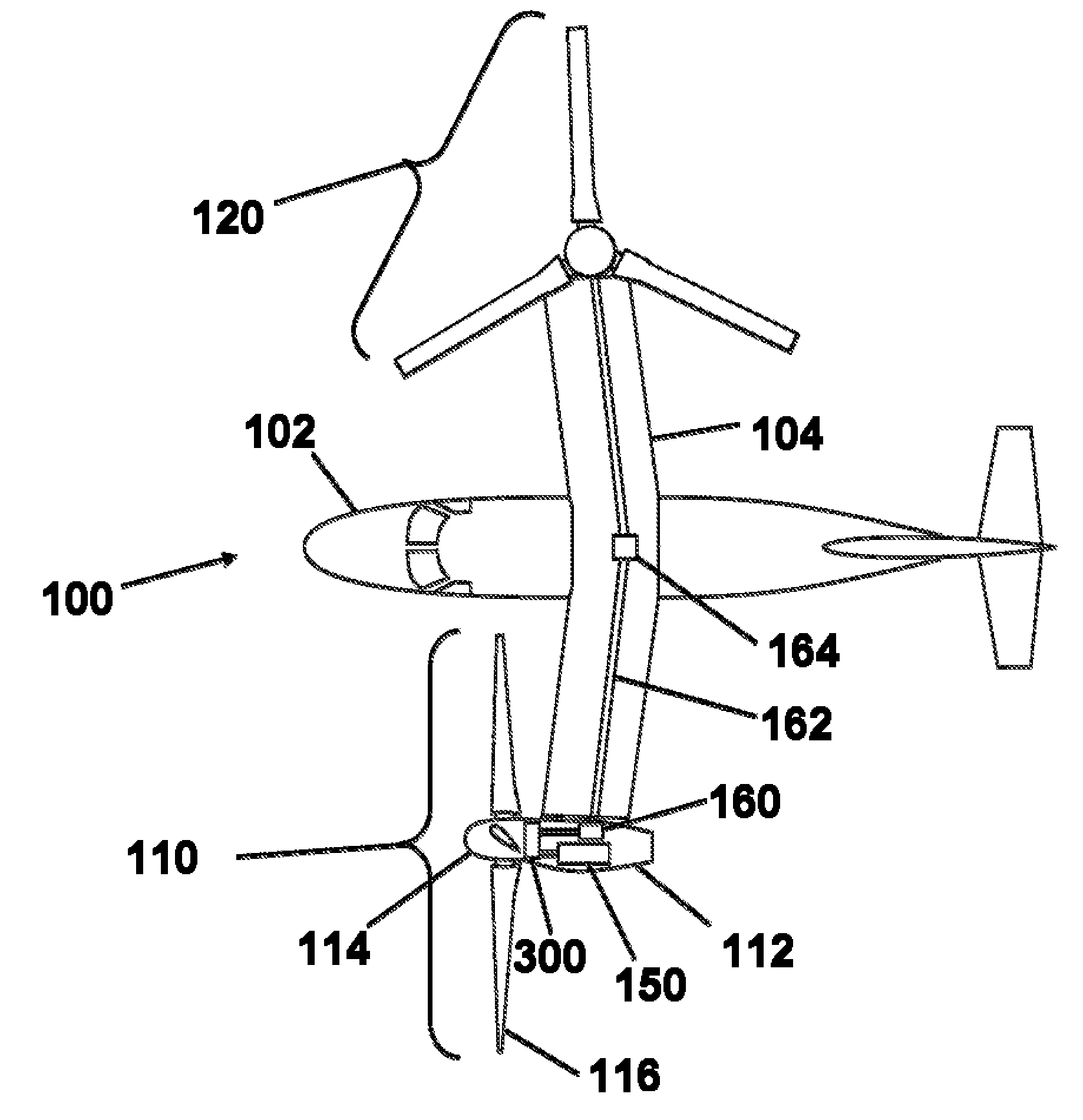
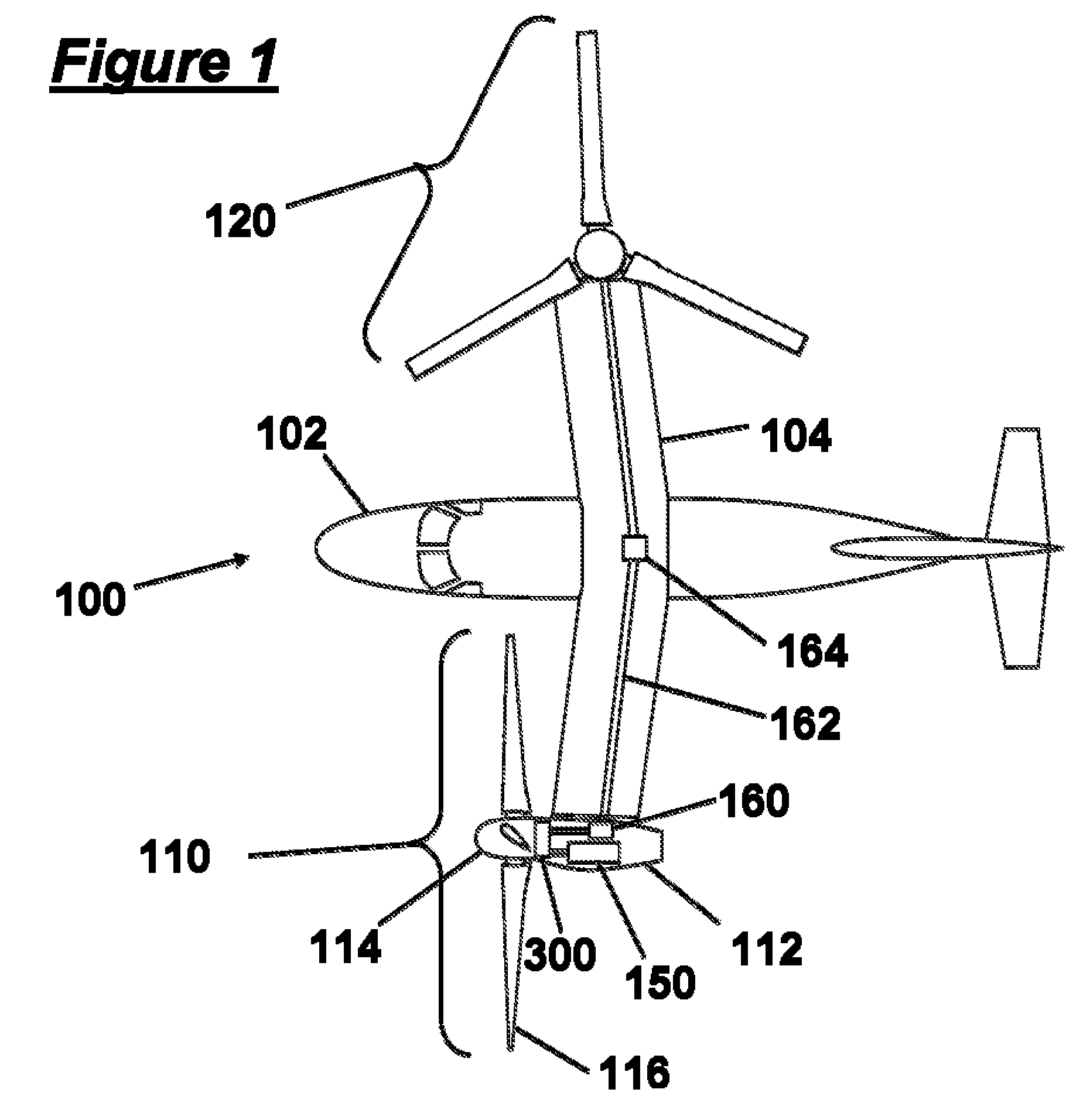
Rotorcraft engine and rotor speed synchronization
ActiveUS20090224096A1Improve reliabilityReduce weightClutchesPower plant arrangements/mountingFlight vehicleRotor speed
Systems and methods are provided in which an electrical control system independently effects acceleration of both driven and driving elements of a clutch to engage each other. In preferred embodiments the clutch is not a friction clutch, but a dog clutch, and forms part of a drive drain of a rotorcraft. A second clutch can be used, along with a mechanical interlock to prevent simultaneous engagement of the clutches. Speeds of the driven and driving elements can be sensed, and altered using at least one of a rotor, a brake, a generator, an electric motor, and a combustion motor. In rotorcraft embodiments, the gearbox can have a neutral condition in which no power is transmitted from the engine to the rotor
Owner:KAREM AIRCRAFT INC
Magnetic metal sensor and method for detecting magnetic metal
InactiveUS6236200B1Easy to separateHigh response rateSolid-state devicesMagnetic field measurement using galvano-magnetic devicesElectrical polarityMagnetic reluctance
A magnetic metal sensor having a high response speed and which can detect small-sized metal pieces and can elongate the separation from the metal pieces. A magnetic metal sensor 2 has a core 22 defining a substantially U-shaped open magnetic path and coils 23, 24 of the same polarity mounted on the core 22. A uniform magnetic field along the direction of magnetic sensitivity is applied by a magnet 25 across the coils 24, 24. If magnetic metal approaches to a open magnetic path portion of the core 22 of the magnetic metal sensor 2, the magnetic reluctance of the magnetic circuit formed by the core and air is changed, as a result of which the impedance of the cores 23, 24 is changed. The magnetic metal sensor 2 detects the possible presence of magnetic metal or its displacement based on impedance changes of the paired coils 23, 24.
Owner:DMG MORI SEIKI CO LTD
Rear wheel hub, in particular for bicycles
ActiveUS7562755B2Readily be remounted and cleaned and reattachedLight weightRotating vibration suppressionYielding couplingFreewheelSprocket
Owner:SWISS
Treatment of depression and pharmaceutical preparations therefor
InactiveUS6191133B1Good curative effectEliminate side effectsBiocideAmine active ingredientsNorepinephrine reuptake inhibitorNoradrenaline reuptake
It has been found that the treatment of depression using known serotonin reuptake inhibitors (SRIs) and noradrenaline reuptake inhibitors (NRIs) may be improved by the administration therewith of folic acid or a precursor which produces folate in the patient. The daily dose of NRI or SRI is as prescribed for treatment of depression in the usual way. The daily dose of the folic acid or precursor should be such as to provide a folate dosage of 300-5000 micrograms / day.
Owner:COPPEN ALEC JAMES
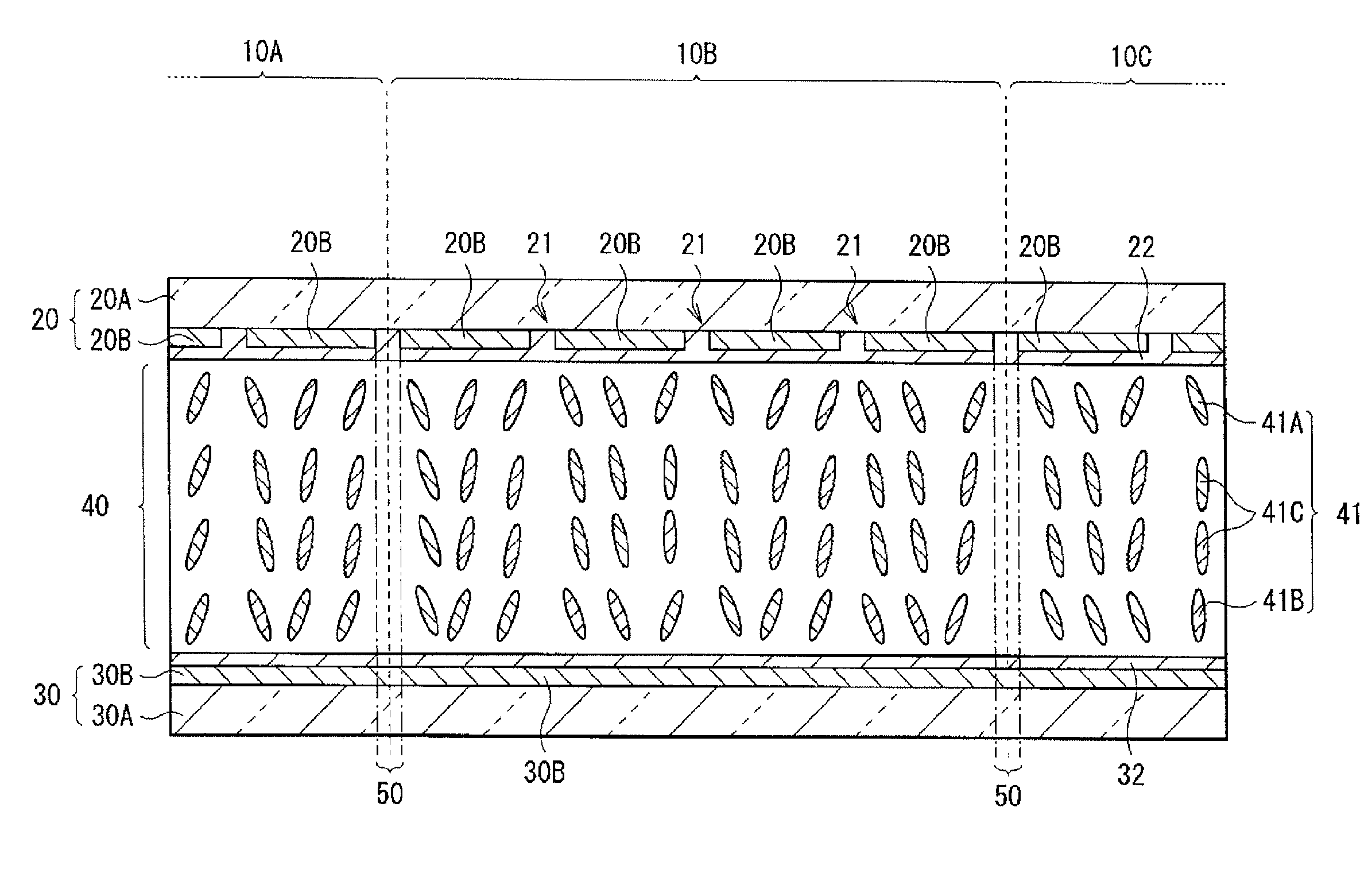
Liquid crystal display unit and method of manufacturing the same
InactiveUS20110157531A1Improve display characteristicsHigh response rateLiquid crystal compositionsLamination ancillary operationsSide chainEngineering
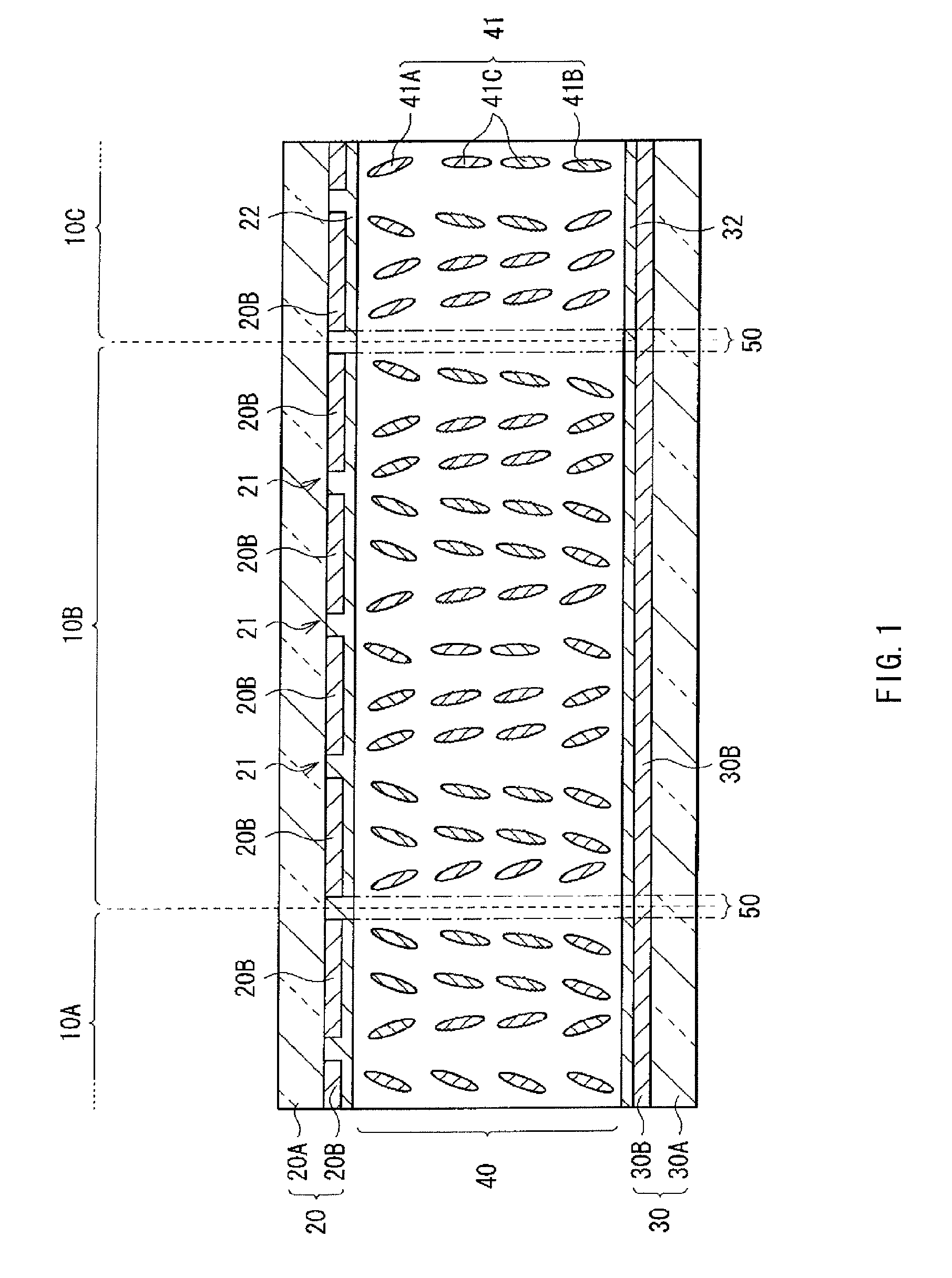
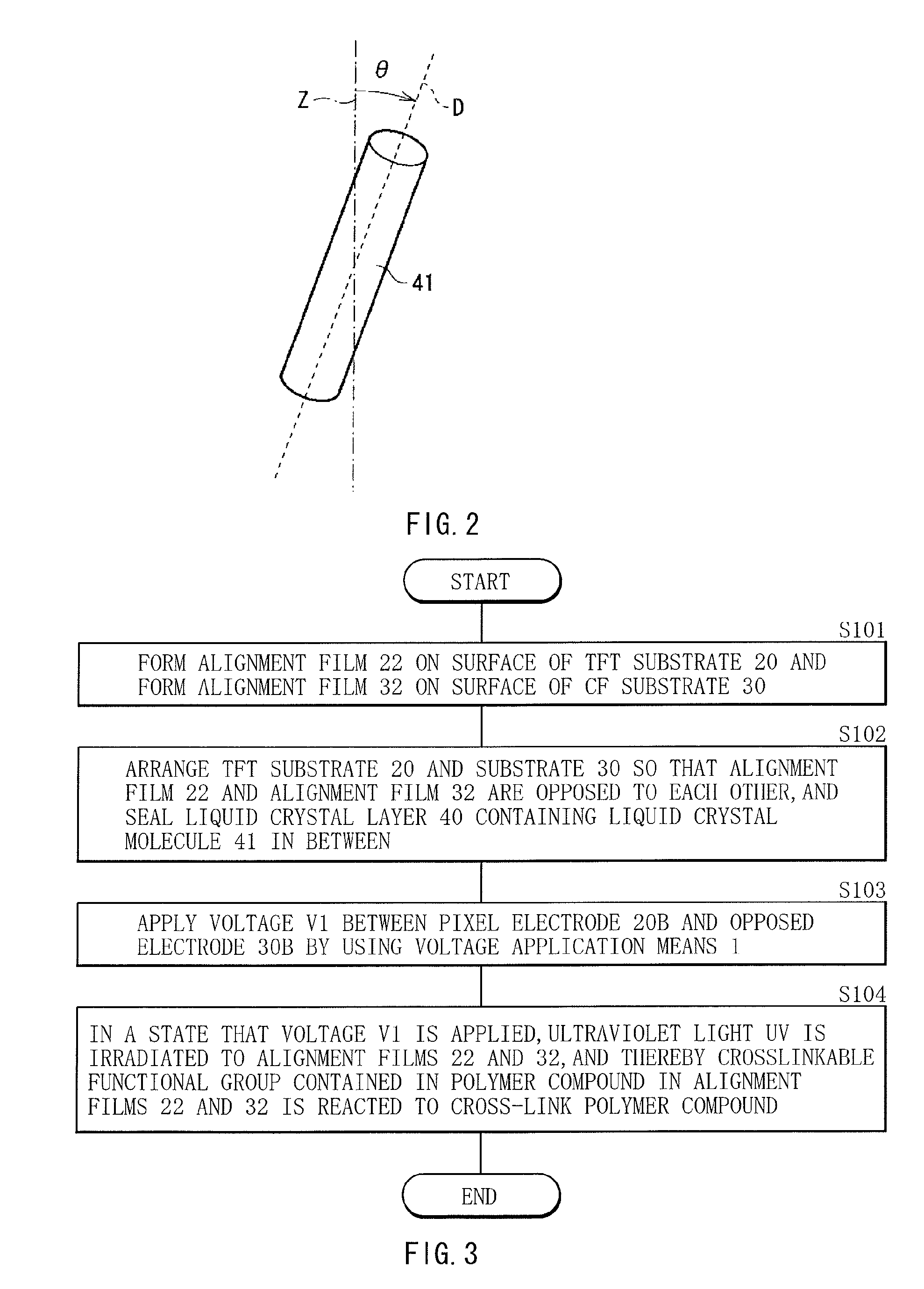
A method of manufacturing a liquid crystal display device with which response characteristics are able to be easily improved without using major equipment is provided. The method of manufacturing a liquid crystal display device includes steps of forming a first alignment film 22 including a polymer compound having a crosslinkable functional group as a side chain on one substrate of a pair of substrates 20 and 30; forming a second alignment film 32 on the other substrate of the pair of substrates 20 and 30; arranging the pair of substrates 20 and 30 so that the first alignment film 22 and the second alignment film 32 are opposed to each other, and sealing a liquid crystal layer 40 containing a liquid crystal molecule 41 having negative dielectric constant anisotropy between the first alignment film 22 and the second alignment film 32; and bridging the polymer compound to give pretilt to the liquid crystal molecule 41 after sealing the liquid crystal layer 40.
Owner:SONY CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com