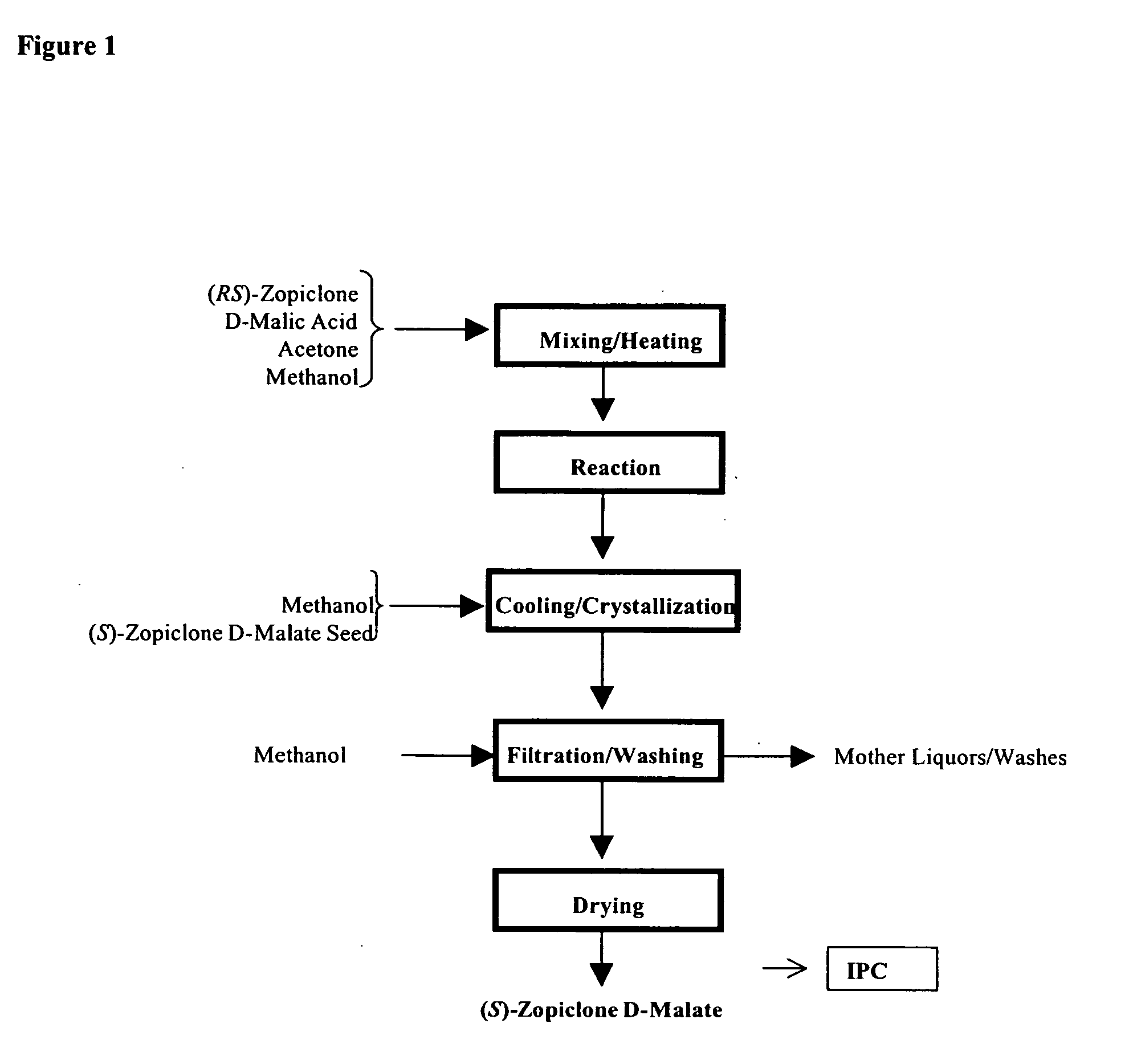
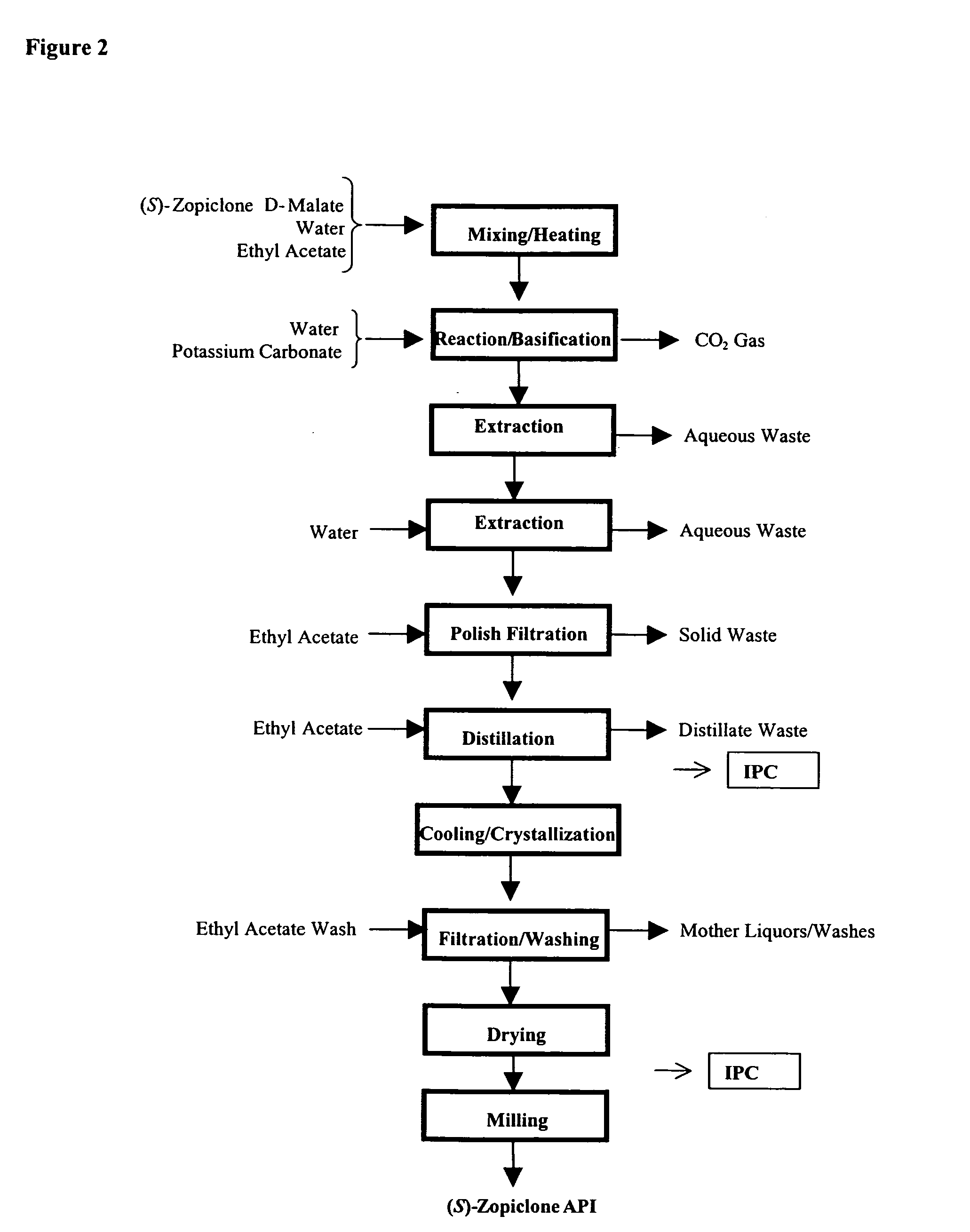
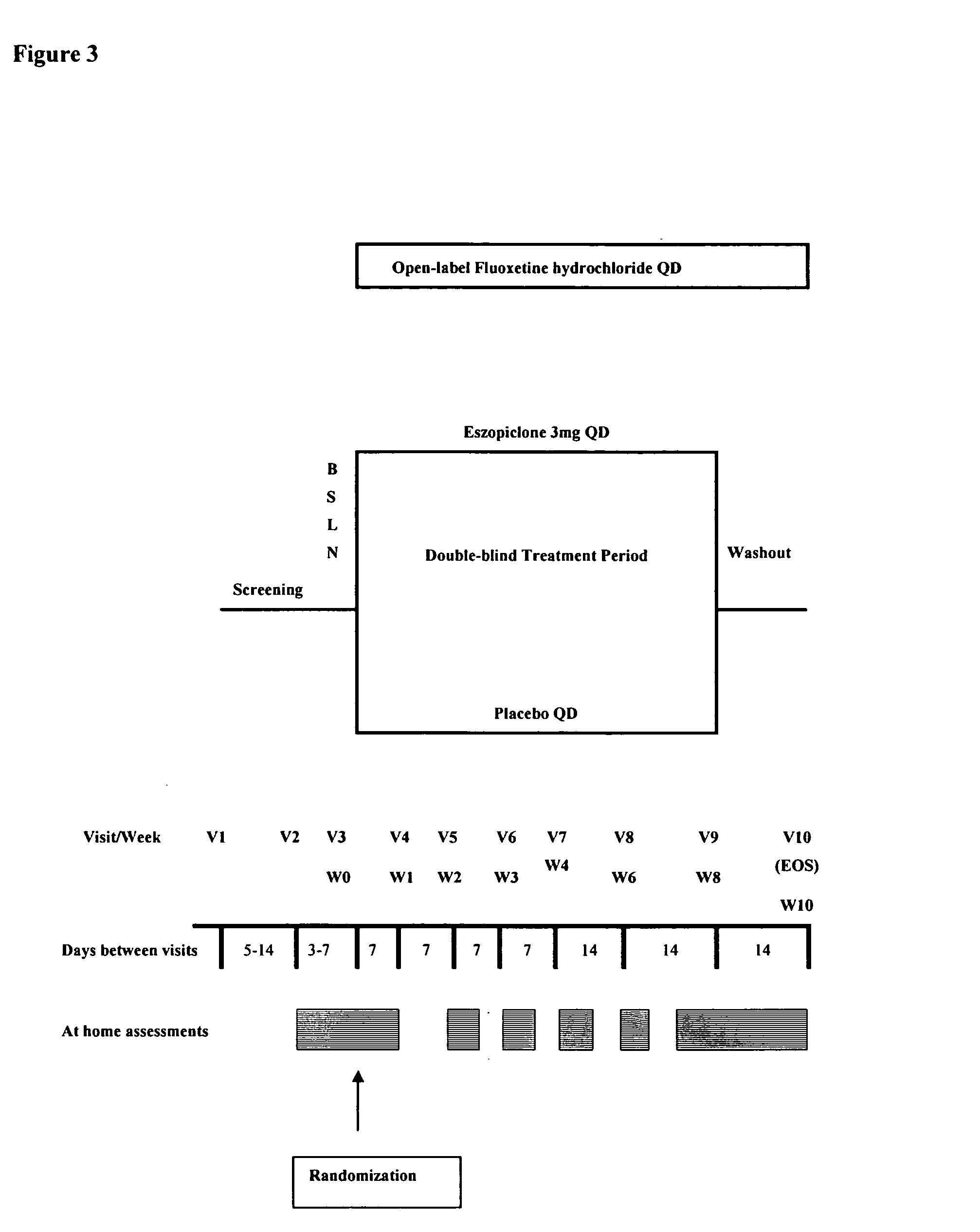
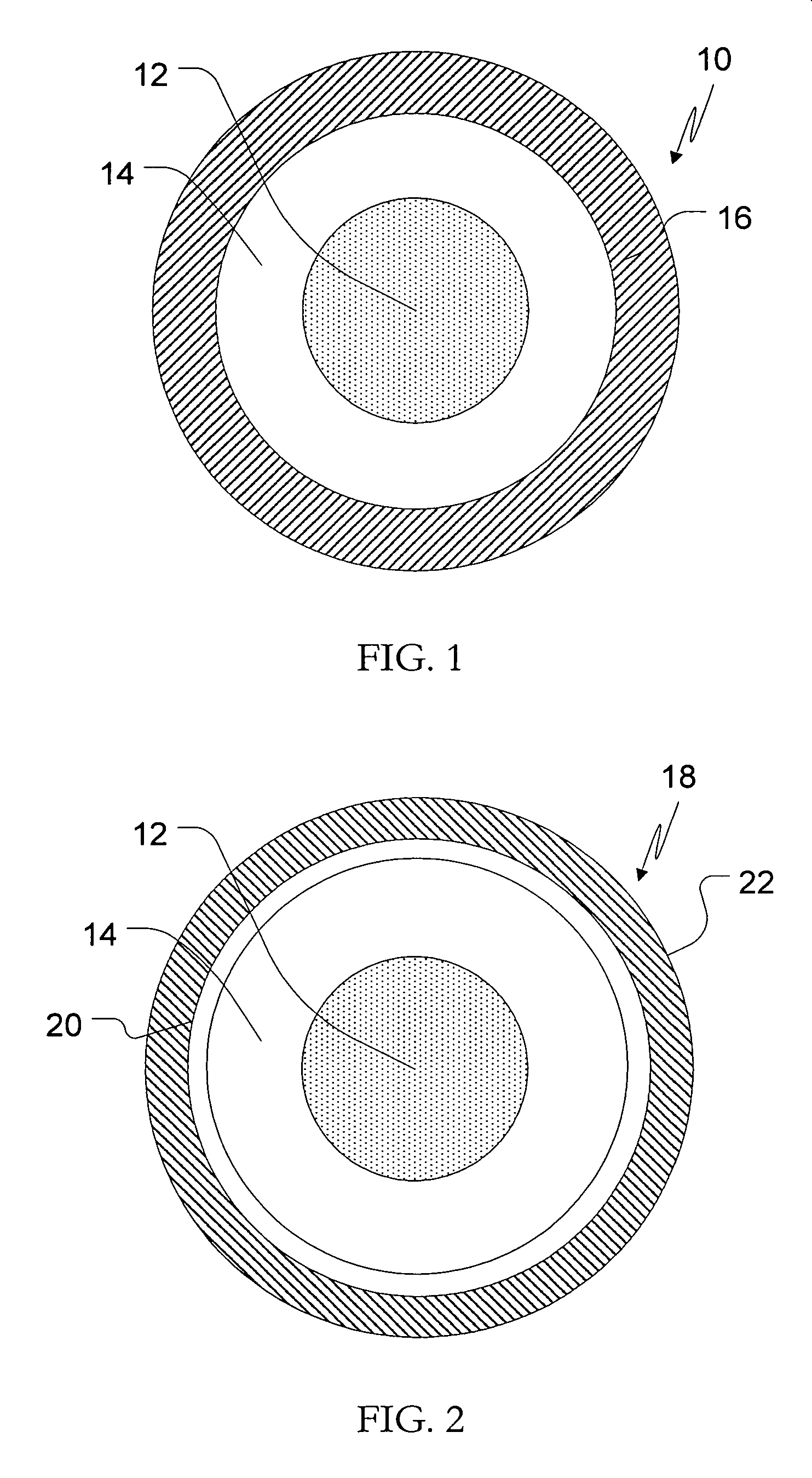
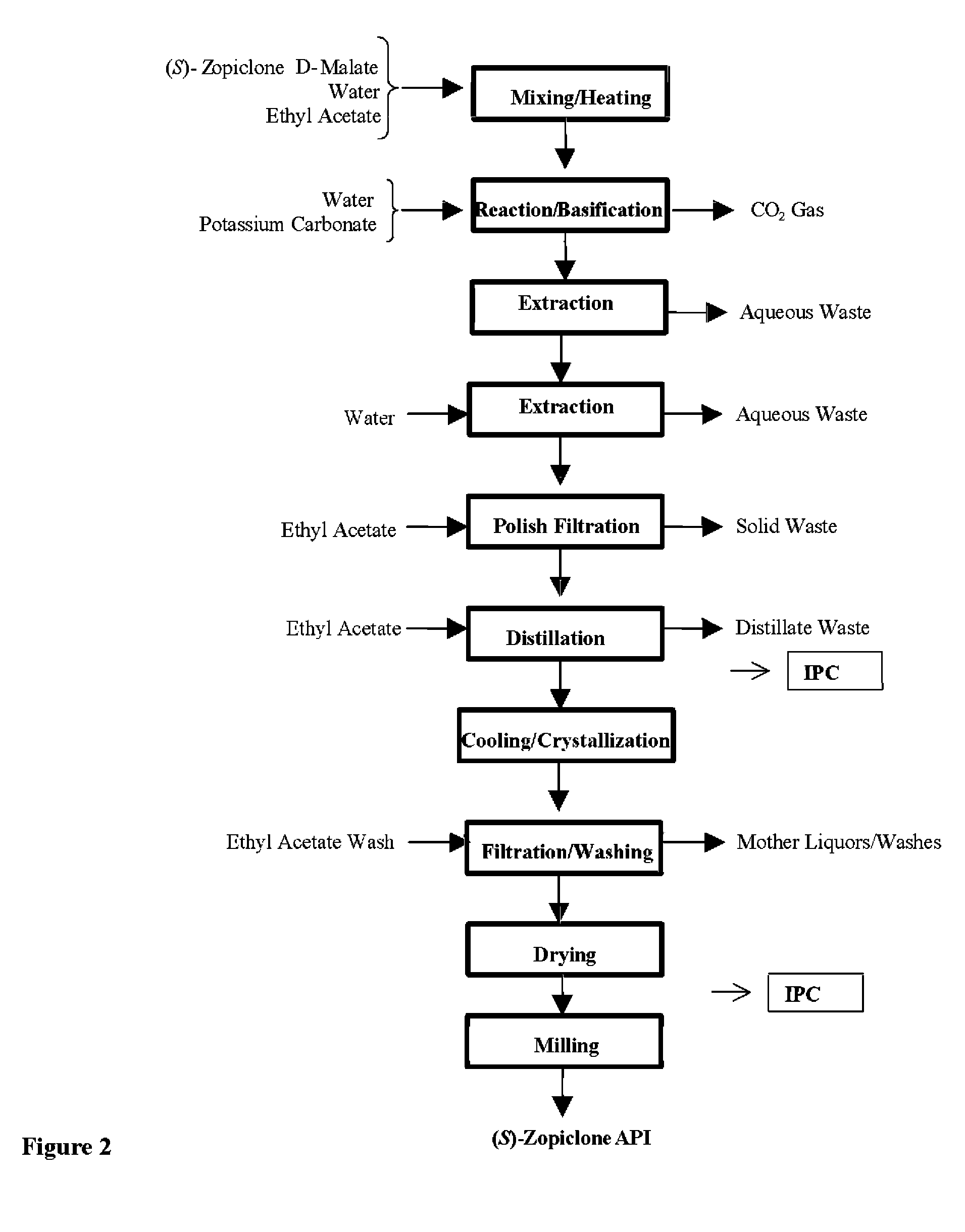
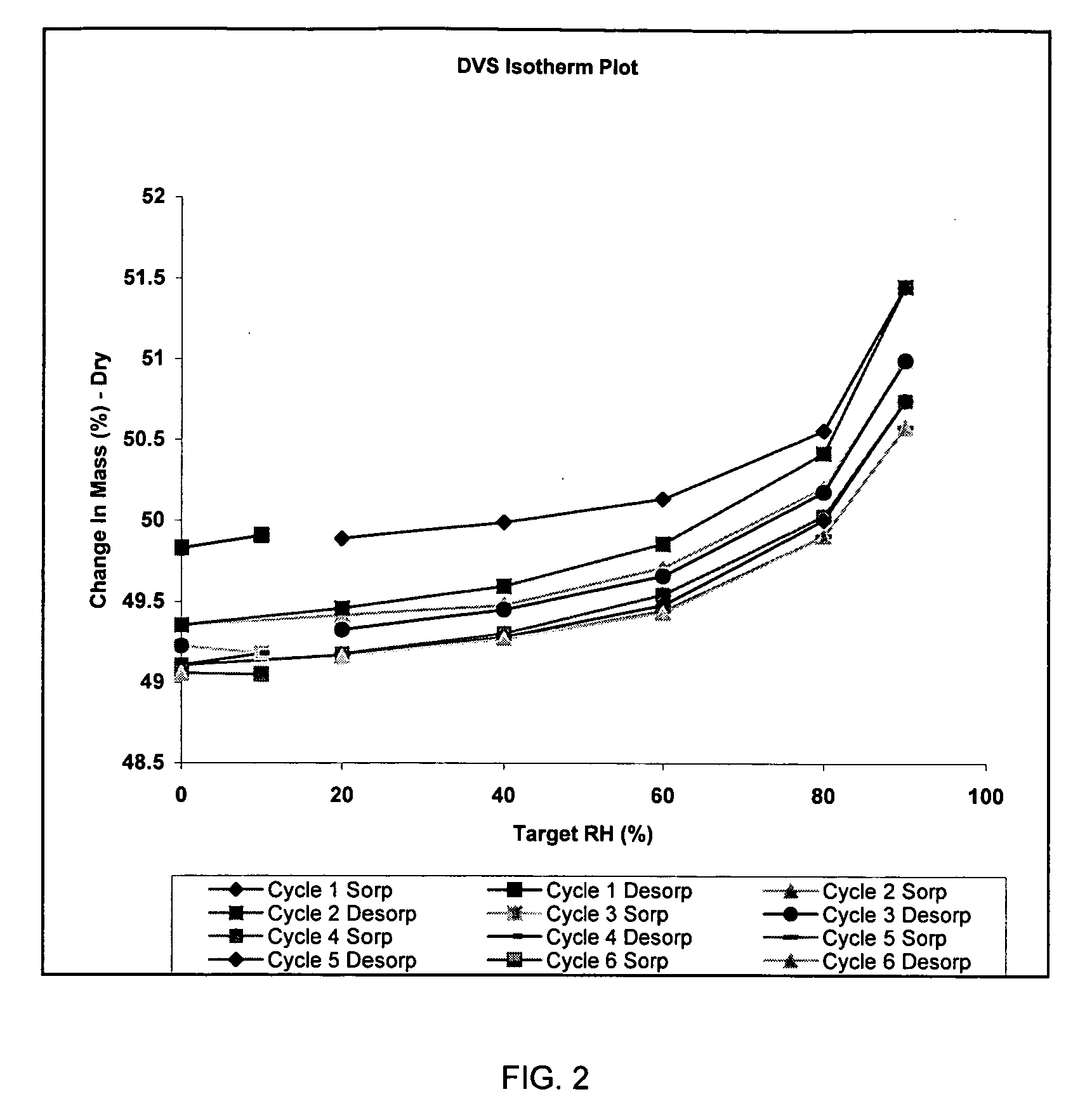
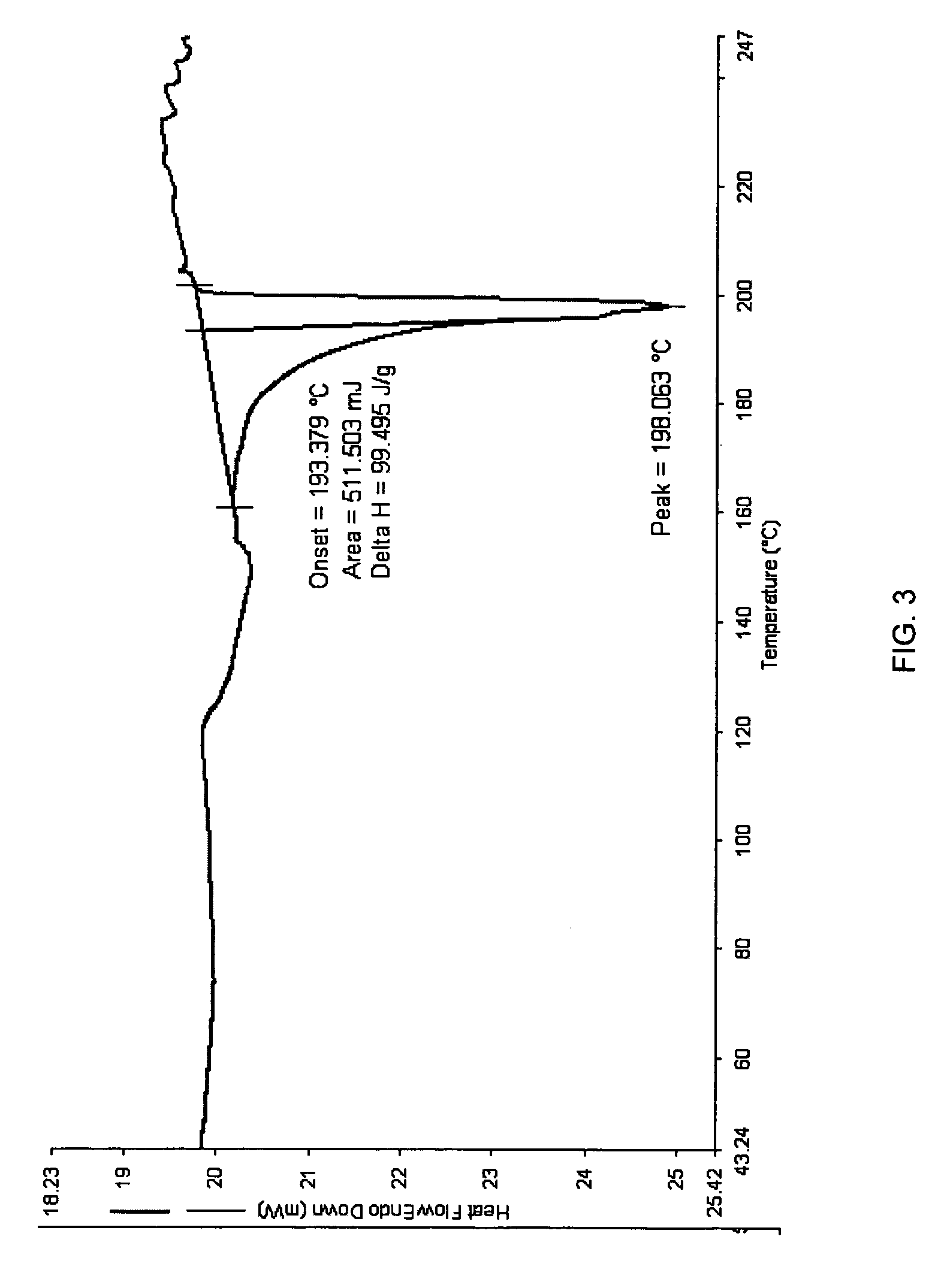
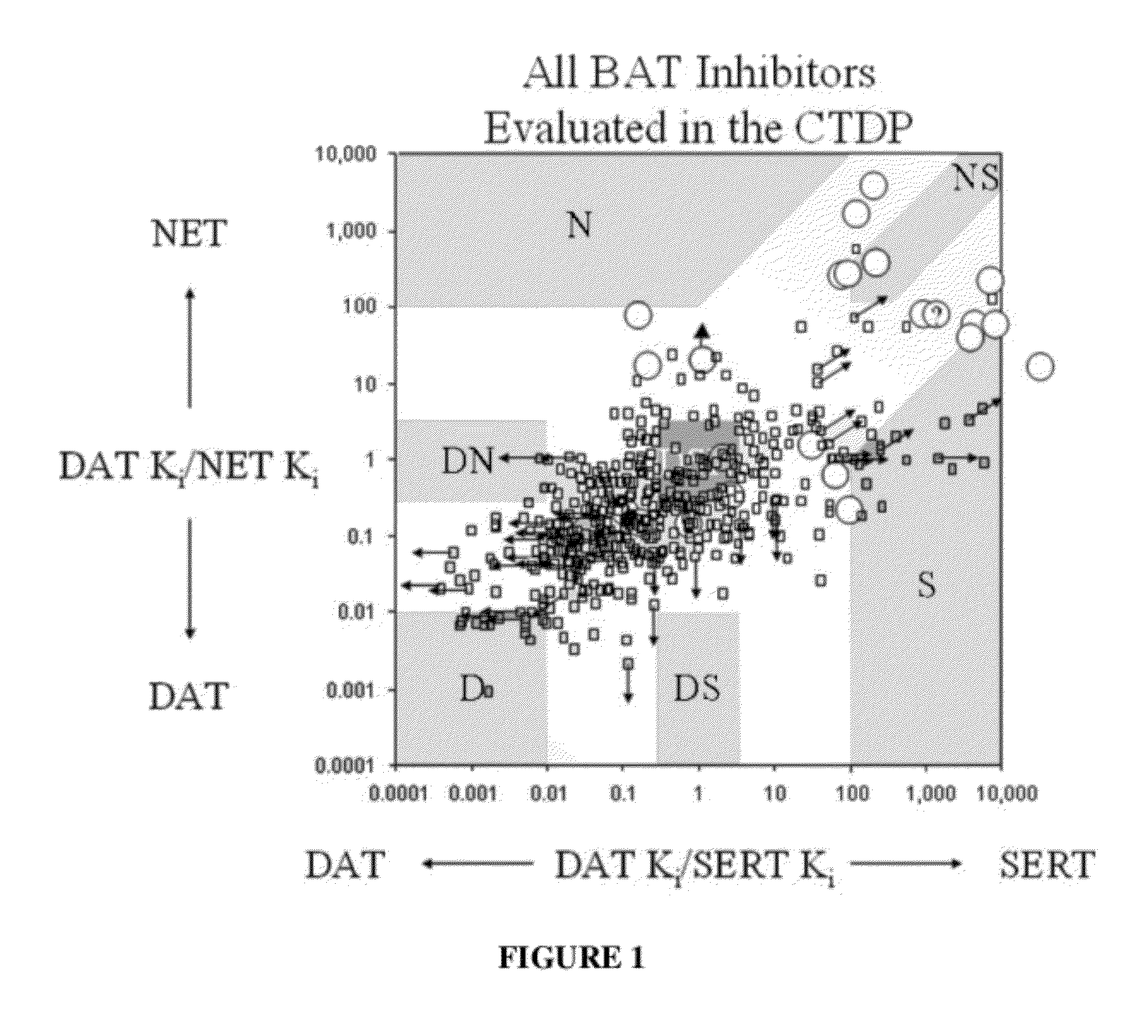
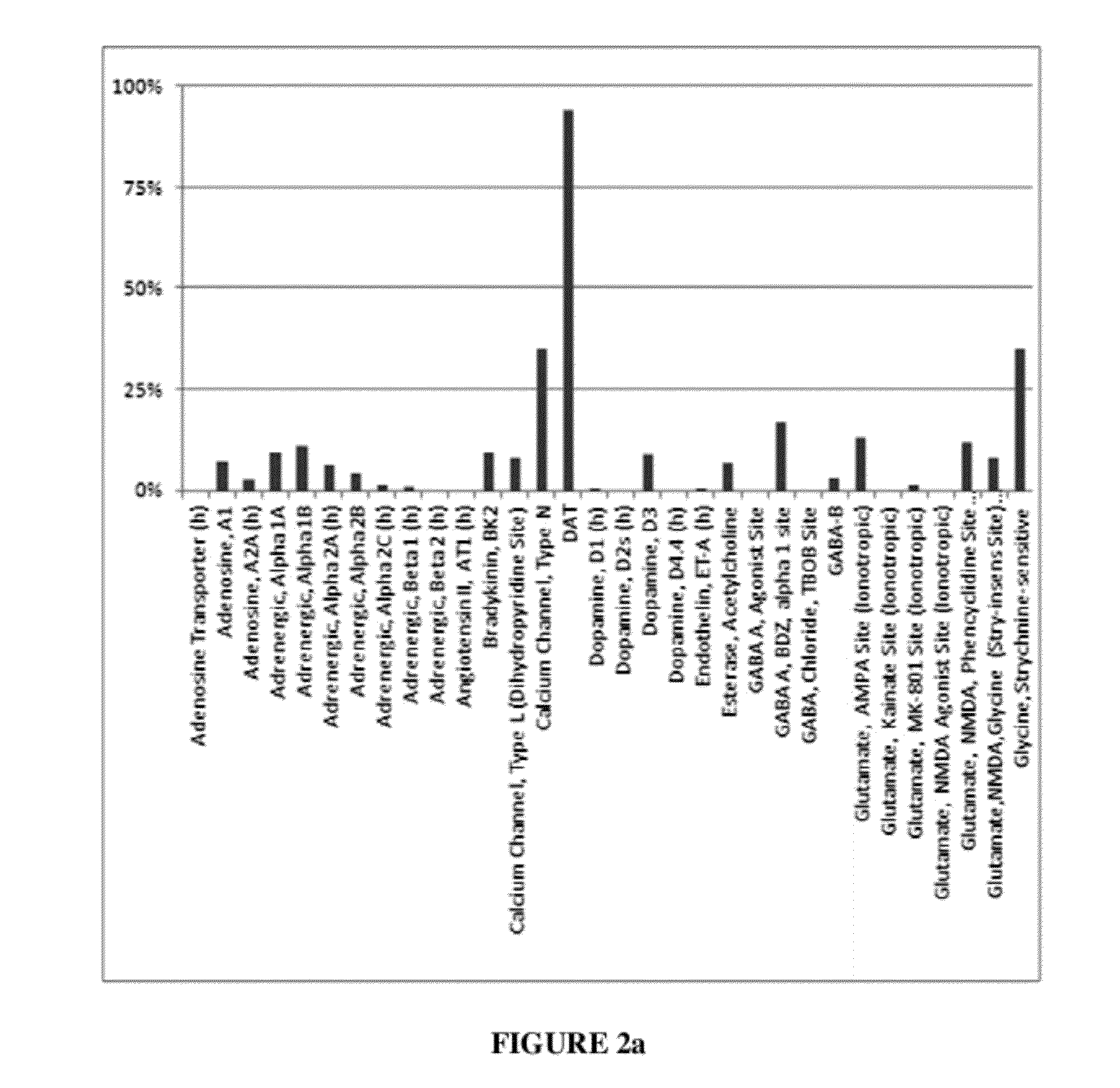
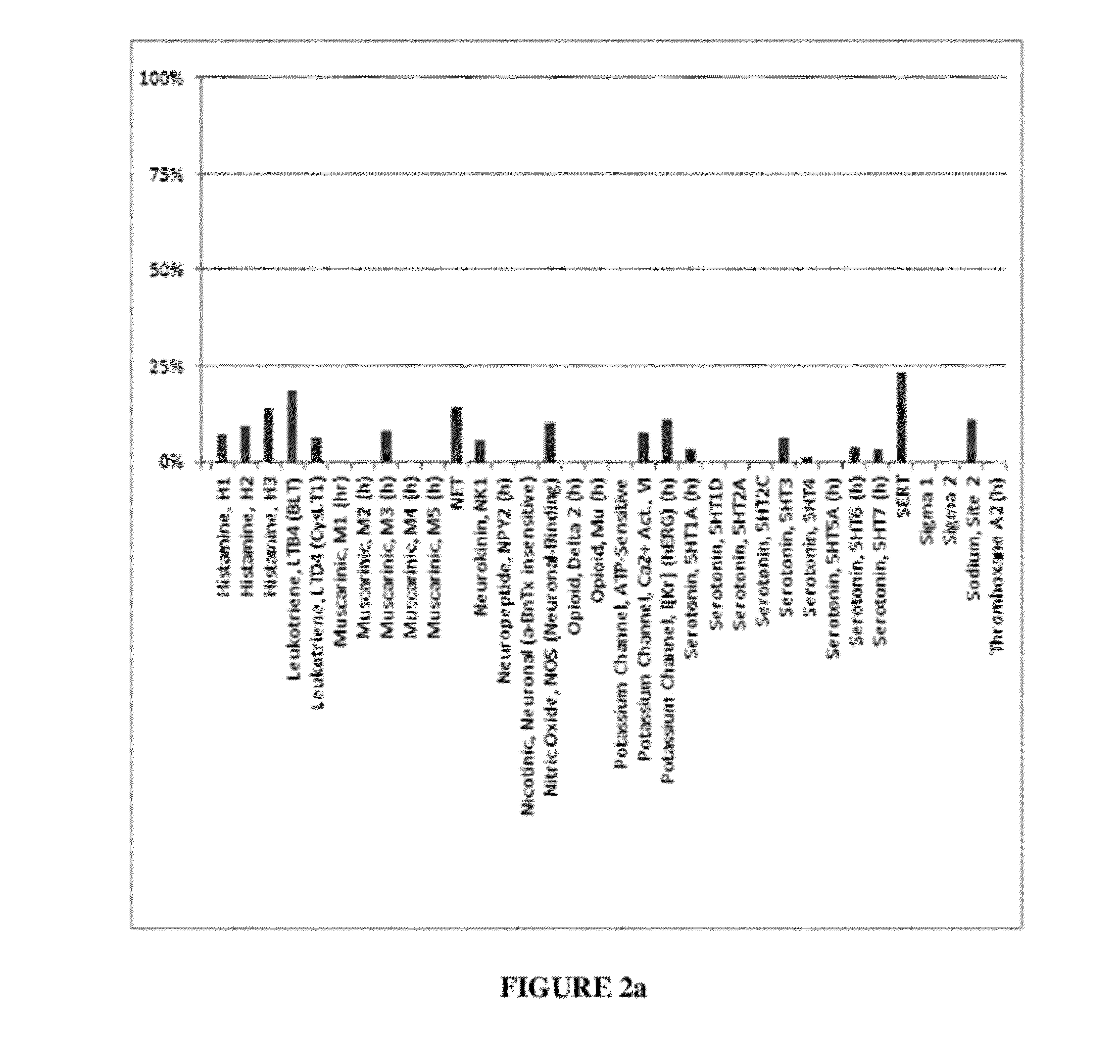
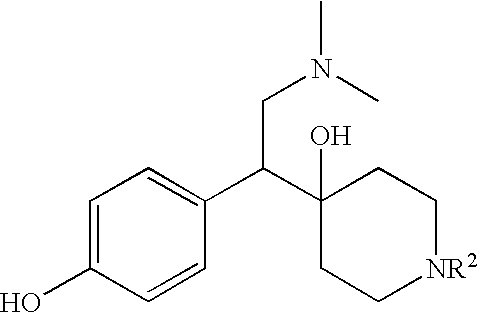
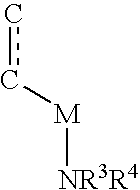Patents
Literature
55 results about "Norepinephrine reuptake inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A norepinephrine reuptake inhibitor (NRI, NERI) or Noradrenaline reuptake inhibitor or adrenergic reuptake inhibitor (ARI), is a type of drug that acts as a reuptake inhibitor for the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline) by blocking the action of the norepinephrine transporter (NET). This in turn leads to increased extracellular concentrations of norepinephrine and epinephrine and therefore can increase in adrenergic neurotransmission.
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:SEPACOR INC
Orally disintegrating tablets of atomoxetine
InactiveUS20060057199A1Low incidenceHighly irritateCosmetic preparationsToilet preparationsOral medicationPopulation
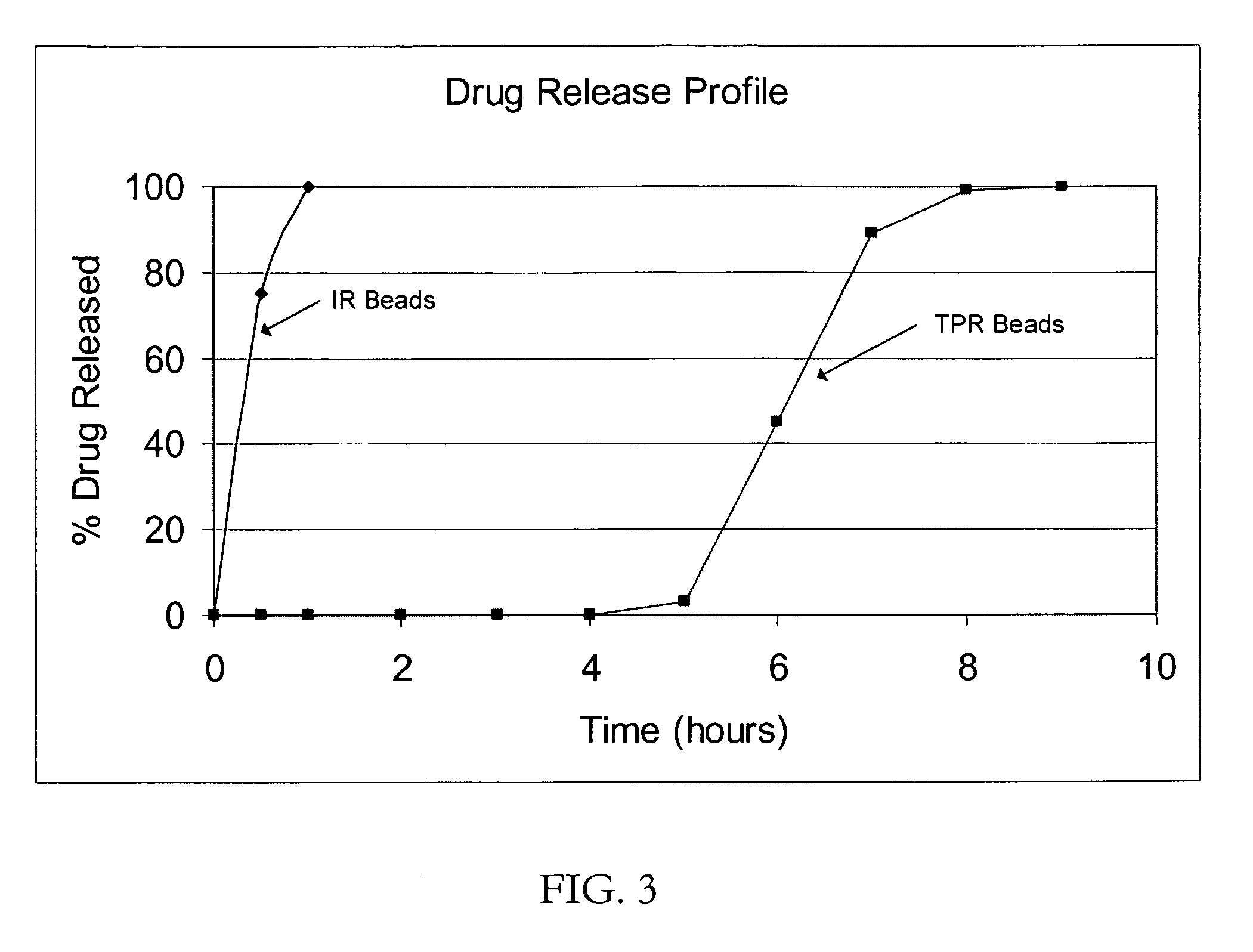
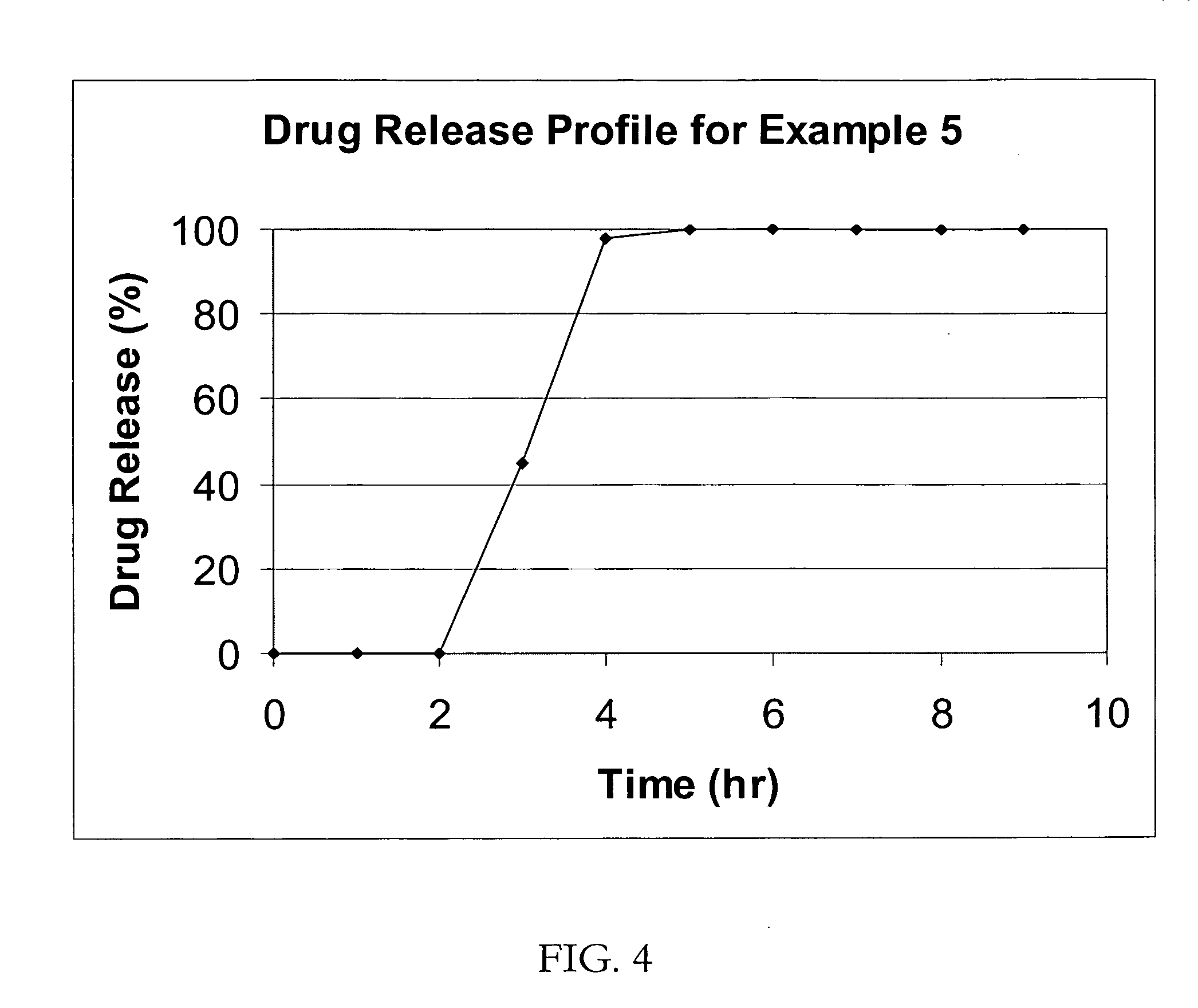
A coated multi-particulate pharmaceutical dosage form such as an orally disintegrating tablet (ODT) presentation for delivering atomoxetine or a pharmaceutically acceptable salt thereof, a selective norepinephrine reuptake inhibitor indicated for the treatment of ADHD, into the body to maintain a therapeutically effective amount of atomoxetine in the plasm. The dosage form may comprise one or more populations of coated atomoxetine-containing particles (beads, pellets, granules etc.) providing a pre-designed rapid release profile after a predesigned lag-time of about 0 to 6 hours following oral administration.
Owner:ADARE PHARM INC
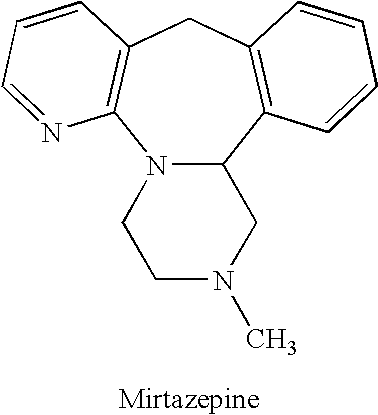
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
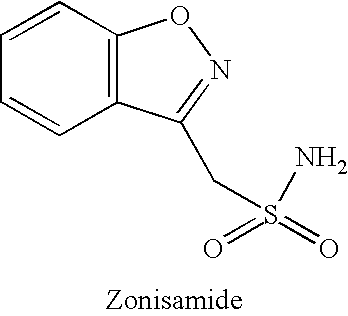
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
Lithium combinations, and uses related thereto
InactiveUS20050233010A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderPsychoactive drugAdrenergic antagonist
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
Treatment of depression and pharmaceutical preparations therefor
InactiveUS6191133B1Good curative effectEliminate side effectsBiocideAmine active ingredientsNorepinephrine reuptake inhibitorNoradrenaline reuptake
It has been found that the treatment of depression using known serotonin reuptake inhibitors (SRIs) and noradrenaline reuptake inhibitors (NRIs) may be improved by the administration therewith of folic acid or a precursor which produces folate in the patient. The daily dose of NRI or SRI is as prescribed for treatment of depression in the usual way. The daily dose of the folic acid or precursor should be such as to provide a folate dosage of 300-5000 micrograms / day.
Owner:COPPEN ALEC JAMES
Combination of serotonin reuptake inhibitors and norephinephrine reuptake inhibitors
InactiveUS20050014848A1Prevent relapseIncreasing and improving neuronal processBiocideAmine active ingredientsStress inducedNorepinephrine reuptake inhibitor
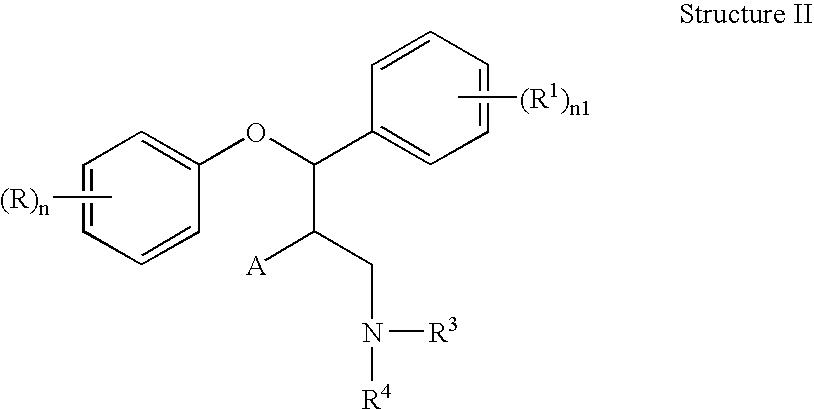
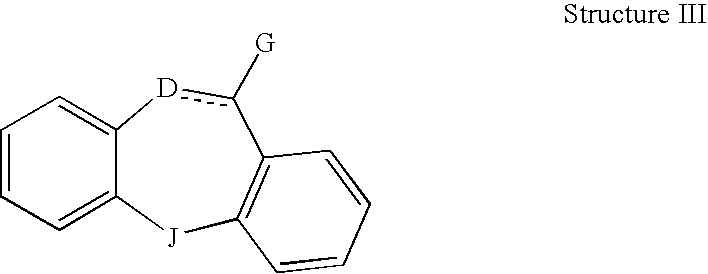
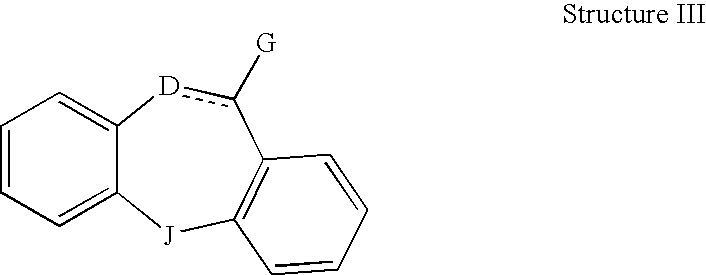
This invention is directed to pharmaceutical compositions and methods for treating a disorder or condition selected from the group consisting of depression, anxiety disorders, phobias, avoidant personality disorder, eating disorders, chemical dependencies, Parkinson's diseases, obsessive-compulsive disorder, negative symptoms of schizophrenia, cognitive dysfunction related to schizophrenia, premenstrual syndrome, stress-induced incontinence, headache, neuropathic pain, chronic pain, urinary incontinence, post-traumatic stress disorder, chronic stress, acute stress, fibromyalgia, depression comorbid with fibromyalgia, obesity, migraine and a combination thereof in a mammal. The methods in one embodiment comprise administering to a mammal in need of treatment for the disorder or condition: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined in the specification; and (iii) a pharmaceutically acceptable carrier. The pharmaceutical compositions and methods of the invention are also useful for preventing a relapse associated with one of the foregoing disorders or conditions, and for treating a symptom associated with one of the foregoing disorders or conditions, wherein the symptom is selected from the group consisting of cognitive dysfunctions and somatic complaints.
Owner:PFIZER INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:WOODWARD SPECIALTY LLC
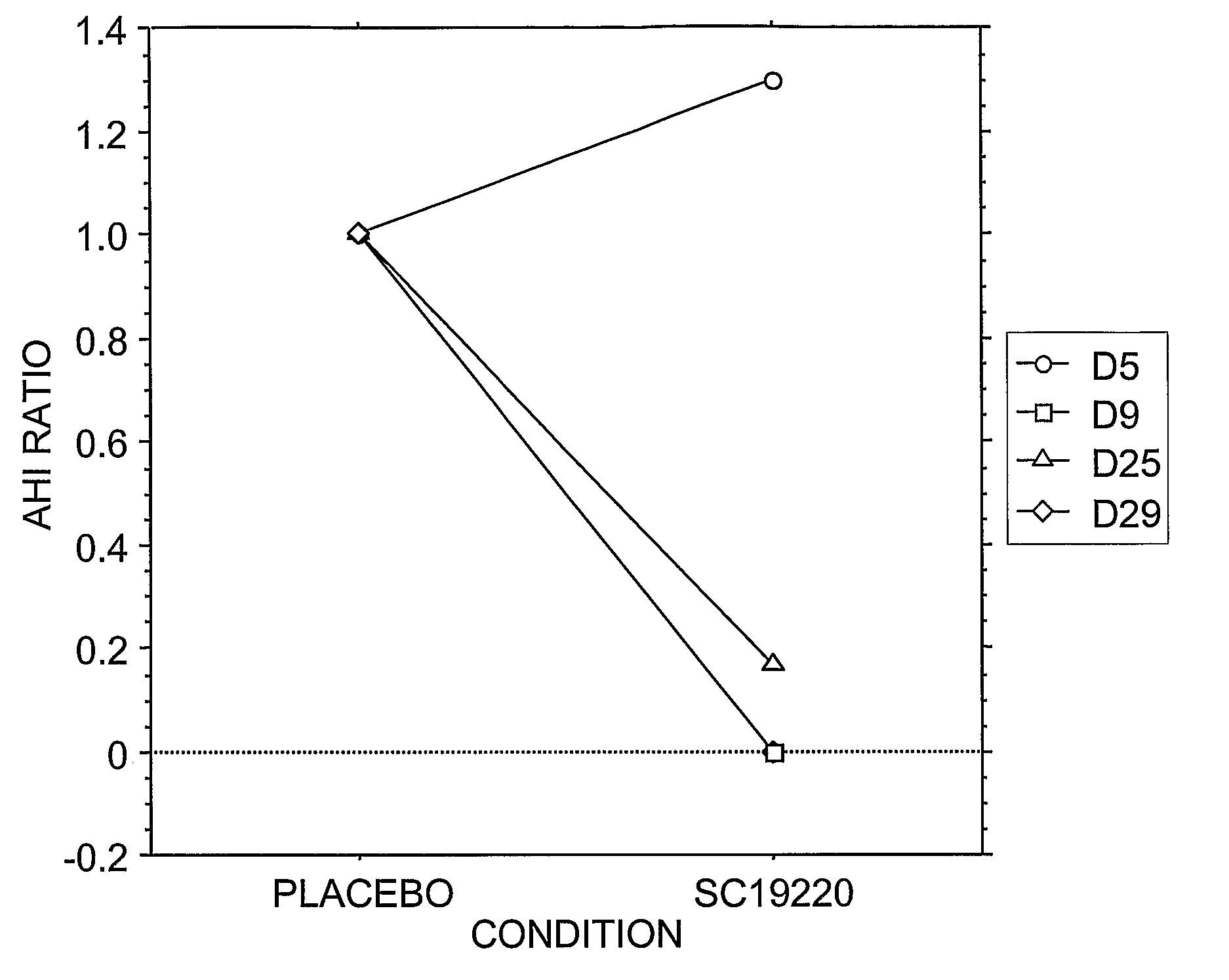
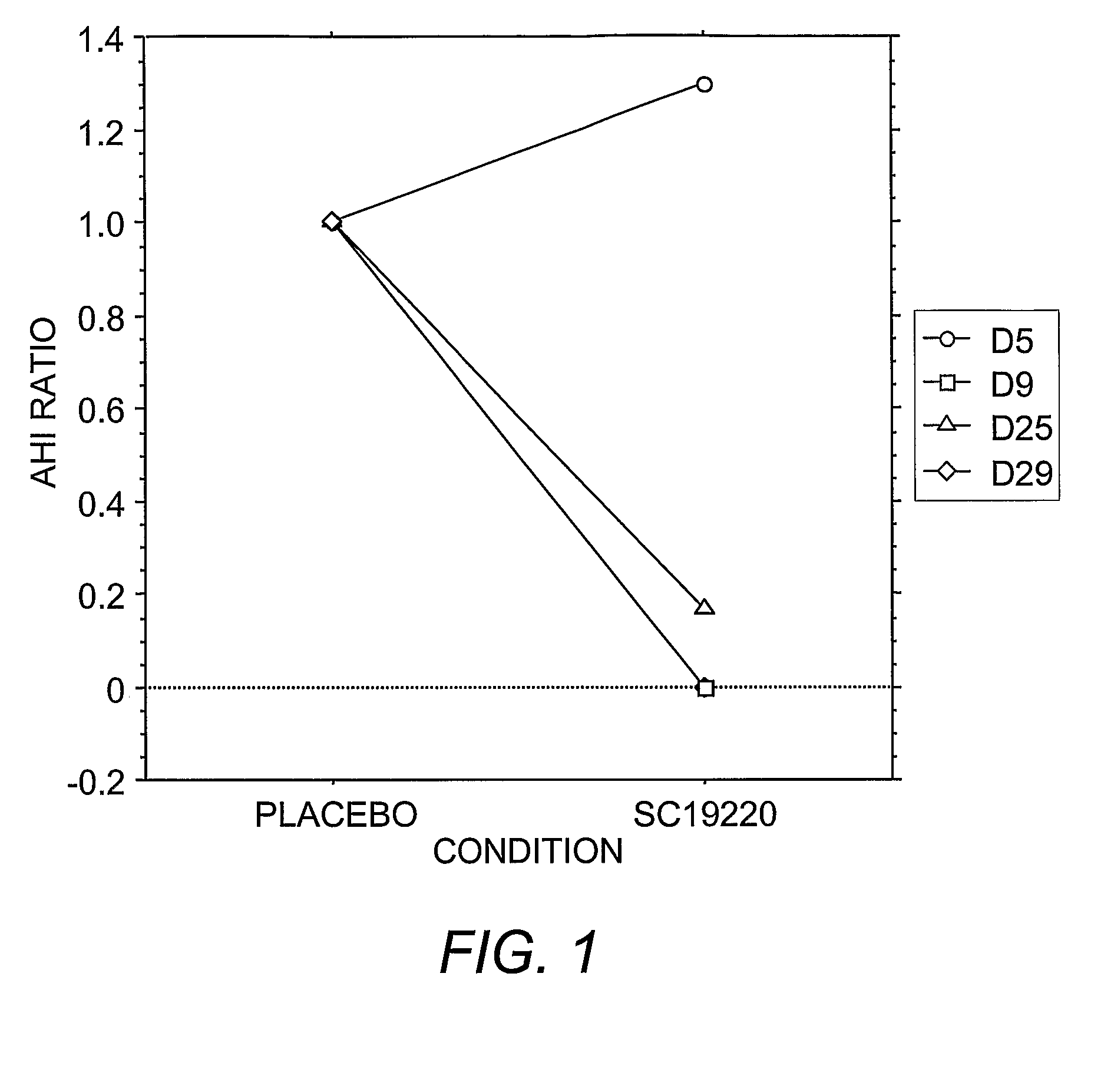
Pharmacological Treatments for Sleep Disorders (Apnoe) With Prostanoid Receptor Antagonists
InactiveUS20080261922A1Preventing and ameliorating sleep-related breathing disorderBiocideNervous disorderNorepinephrine reuptake inhibitorPhosphate
This invention is directed to methods for preventing or ameliorating sleep-related breathing disorders. The method comprises administration to a patient in need thereof an effective dose of one or a combination of prostanoid receptor antagonists (eg. ramatroban, ifetroban, diphloretin, phosphate, polyphloretin phosphate, seratrodast, SC19220). The prostanoid receptor antagonist or combination of prostanoid receptor antagonists can be administered in conjunction with one or more serotonin receptor agonists, one or more cannabinoids receptor agonists, one or more serotonin reuptake inhibitors, one or more noradrenalin reuptake inhibitors, a combination of reuptake inhibitors, an inhibitor of prostanoid synthesis, or any combination of the foregoing.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Multiparticulate selective serotonin and norepinephrine reuptake inhibitor formulation
InactiveUS20100040680A1Protect the surfaceFlat surfacePowder deliveryOrganic active ingredientsNorepinephrine reuptake inhibitorAdrenergic
A multiparticulate oral pharmaceutical composition that contains a plurality of delayed release coated selective serotonin and norepinephrine reuptake inhibitor particles.
Owner:IMPAX LAB INC
Treatment of disorders secondary to organic impairments
A method for treatment of neuropsychiatric symptoms or disorders emanating from primary brain or systemic impairments includes administration of an effective dose of a dopamine, serotonin, and norepinephrine reuptake inhibitor to a human in need of such treatment. The preferred reuptake inhibitor is sibutramine.
Owner:SNOWDEN PHARMA
Highly selective serotonin and norepinephrine dual reuptake inhibitor and use thereof
InactiveUS20070015828A1Eliminate side effectsBiocideNervous disorderNorepinephrine reuptake inhibitorVisceral pain
Highly selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agorophobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, visceral pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
Combination of serotonin reuptake inhibitors and norepinephrine reuptake inhibitors
InactiveUS20050009927A1Inhibits dopamine reuptakeBiocideAnimal repellantsNorepinephrine reuptake inhibitorAdrenergic
This invention is directed in one embodiment to pharmaceutical compositions and methods for treating depression in a mammal. To a mammal in need of such treatment are administered: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; and (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined herein.
Owner:PFIZER INC
Lithium combinations, and uses related thereto
InactiveUS20080107756A1Prevent precipitating manic episodeLessening and preventing riskBiocideNervous disorderNorepinephrine reuptake inhibitorPsychoactive drug
The present invention relates to combinatorial therapies for treating anxiety, depression or psychotic conditions using a lithium salt and a psychoactive drug selected from the group consisting of serotonin reuptake inhibitor, a 5HT2 receptor antagonist, an anticonvulsant, a norepinephrine reuptake inhibitor, an α-adrenoreceptor antagonist, an NK-3 antagonist, an NK-1 receptor antagonist, a PDE4 inhibitor, an Neuropeptide Y5 Receptor Antagonists, a D4 receptor antagonist, a 5HT1A receptor antagonist, a 5HT1D receptor antagonist, a CRF antagonist, a monoamine oxidase inhibitor, a sedative-hypnotic drug, and an atypical antipsychotic.
Owner:NOVEN THERAPEUTICS
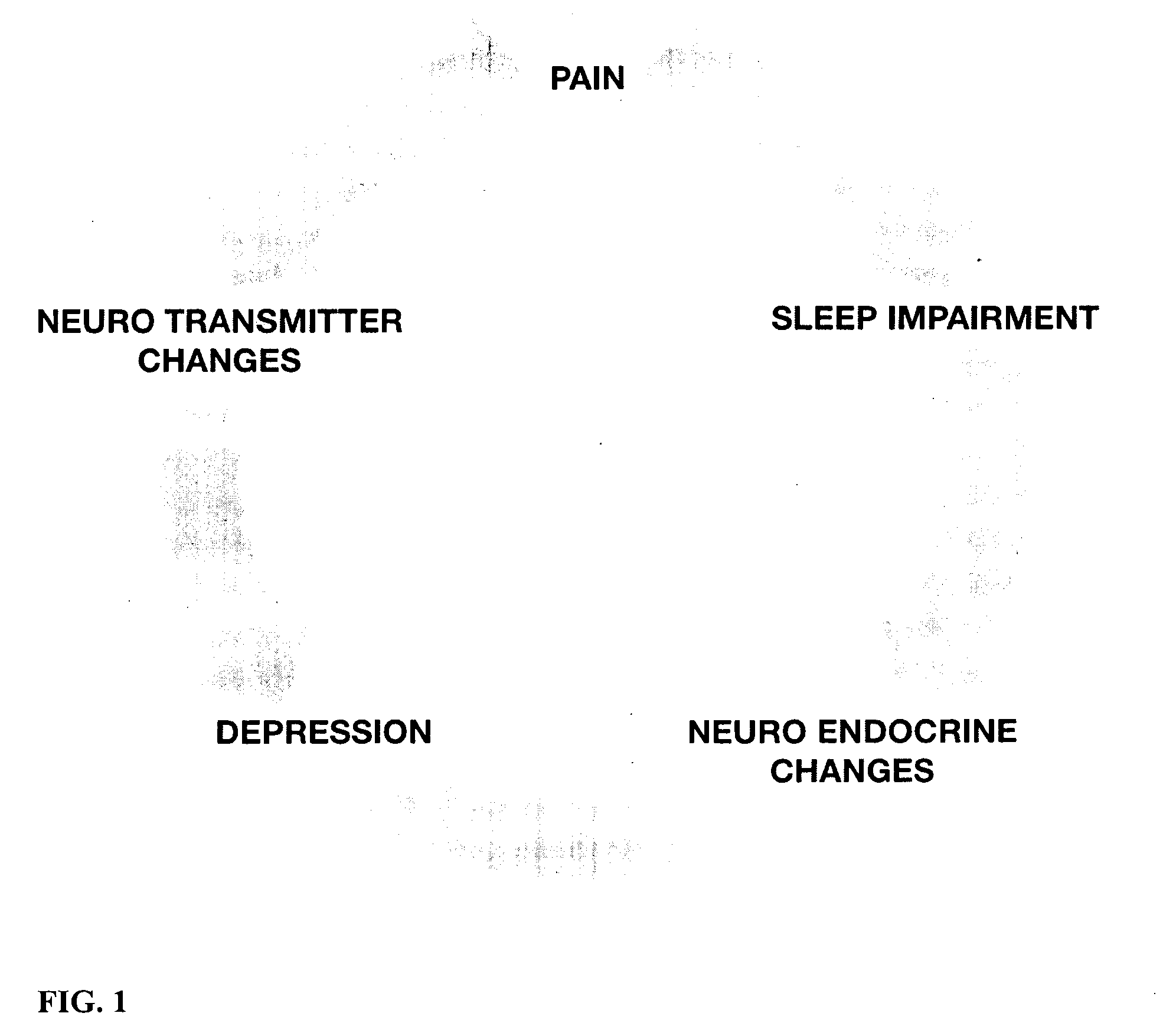
Prevention and treatment of functional somatic disorders, including stress-related disorders
InactiveUS20090105222A1Improve concentrationBiocideNervous disorderNorepinephrine reuptake inhibitorStress-related disorders
Methods for the prevention or treatment of stress-related disorders by administering a therapeutically effective amount of a dual serotonin / norepinephrine reuptake inhibitor to an individual under stress are described. A triple monoamine reuptake inhibitor for serotonin / noradrenaline / dopamine may also be administered to an individual at risk for a stress-related disorder. In a preferred embodiment the compound is milnacipran and is prophylactically administered at an effective amount to delay or prevent stress-related disorders in an individual at risk.
Owner:CYPRESS BIOSCI
Methods of treating vasomotor symptoms
InactiveUS20050130987A1BiocideAmine active ingredientsNorepinephrine reuptake inhibitorVasomotor symptom
The present invention relates to selective adrenergica2B antagonists alone, selective adrenergicα2B antagonists in combination with norepinephrine reuptake inhibitors (NRI) (as a single compound or as a combination of two or more compounds), or selective adrenergicα2B antagonists in combination with dual norepinephrine reuptake inhibitors / serotonin reuptake inhibitors (NRI / SRI) (as a single compound or as a combination of two or more compounds) and methods of their use in the treatment of vasomotor symptoms.
Owner:WYETH LLC
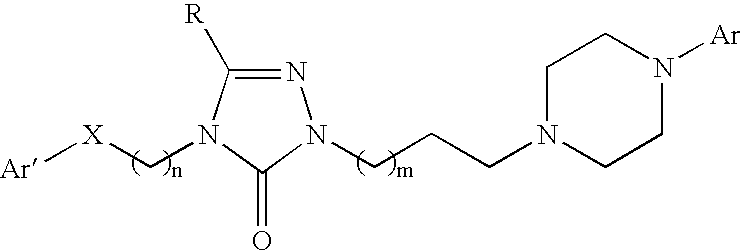
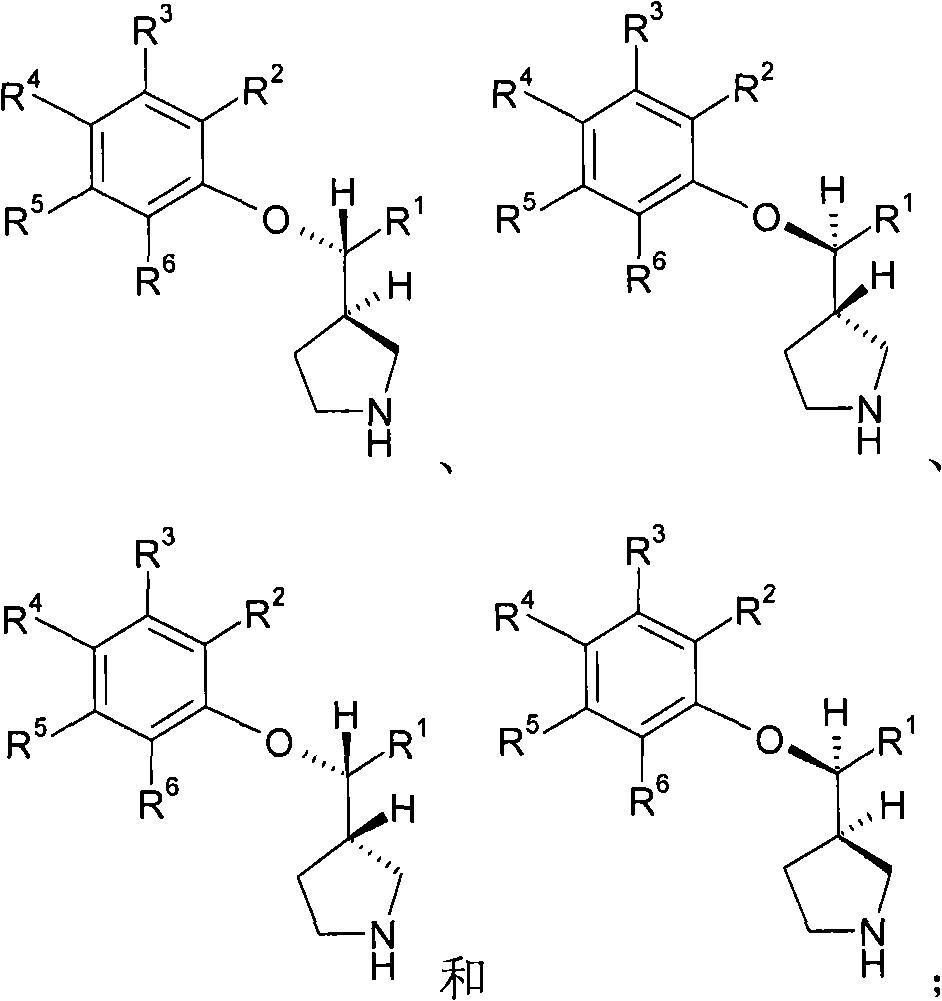
3-(phenoxyphenylmethyl)pyrrolidine compounds
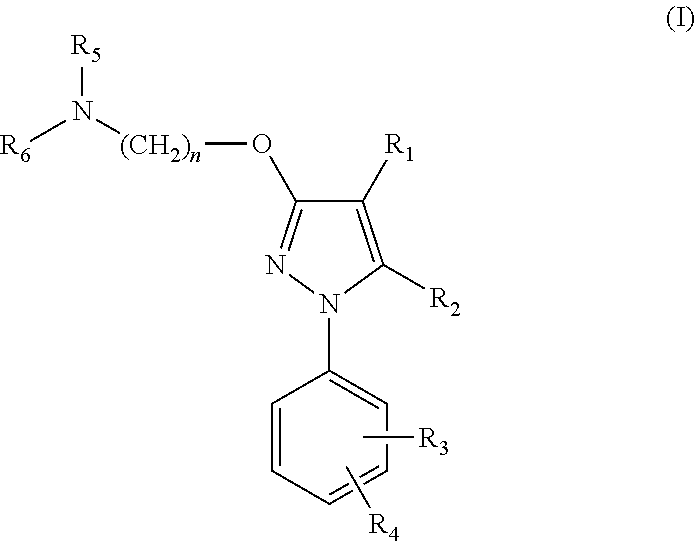
In one aspect, the invention relates to compounds of formula I:where a and R1-6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. The compounds of formula I are serotonin and norepinephrine reuptake inhibitors. In another aspect, the invention relates to pharmaceutical compositions comprising such compounds; methods of using such compounds; and process and intermediates for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Method of using dopamine reuptake inhibitors and their analogs for treating diabetes symptoms and delaying or preventing diabetes-associated pathologic conditions
ActiveUS9119843B2Attenuate diabetic neurologicalLow extremityBiocideMetabolism disorderAcute hyperglycaemiaNorepinephrine reuptake inhibitor
Method of using dopamine reuptake inhibitors, e.g., sydnonimine derivatives, for the management of diabetic symptoms and associated complications or conditions, such as hyperglycemia and diabetic neuropathy.
Owner:CAPLIPER LIFE SCI INC
Serotonin and norepinephrine reuptake inhibitor and uses thereof
InactiveUS20070015824A1Low level of undesirable side-effectsBiocideOrganic chemistryNorepinephrine reuptake inhibitorDepressant
Selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agoraphobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH
Method of using dopamine reuptake inhibitors and their analogs for treating autoimmune conditions and delaying or preventing autoimmune related pathologic progressions
ActiveUS20120264698A1Effective therapeutic approachBiocideNervous disorderNorepinephrine reuptake inhibitorDisease
Owner:CAPLIPER LIFE SCI INC
Serotonin and norepinephrine reuptake inhibitors and uses thereof
InactiveUS20070015791A1Reduce adverse side effectsBiocideOrganic chemistryNorepinephrine reuptake inhibitorS syndrome
Selective dual serotonin and norepinephrine reuptake inhibitors are provided. These compounds have a lower side-effect profile and are useful in compositions and products for use in treatment of a variety of conditions including depression, fibromyalgia, anxiety, panic disorder, agorophobia, post traumatic stress disorder, premenstrual dysphoric disorder, attention deficit disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, autism, schizophrenia, obesity, anorexia nervosa, bulimia nervosa, Gilles de la Tourette Syndrome, vasomotor flushing, cocaine and alcohol addiction, sexual dysfunction, borderline personality disorder, fibromyalgia syndrome, diabetic neuropathic pain, chronic fatigue syndrome, pain, Shy Drager syndrome, Raynaud's syndrome, Parkinson's Disease, and epilepsy.
Owner:WYETH LLC
Method for treating neurasthenia or somatic form disorders and medicinal composition
ActiveCN1850271AOrganic active ingredientsSolution deliveryNorepinephrine reuptake inhibitorSertraline
The present invention relates to a method for curing and preventing neurosism or somatic formal disturbance and its medicine composition. Said invention belongs to the field of pharmacy. Said invention provides a medicine composition containing norepinephrine reuptake inhibitor with medicinal dose or its medicinal salt and selective 5-hydroxytryptamine reuptake inhibitor with medicinal dose or its medicinal salt for preventing and curing neurosism or somatic formal disturbance. The norepinephrine reuptake inhibitor is selected from reboxetine, tomoxetine, amfebutamone, nortriptyline, norimipramine, maprotiline and protiline, and the selective 5-hydroxytryptamine reuptake inhibitor is selected from sertraline, citalopram, s-citalopram, fluoroxeline, paroxetine and fluvoxamine.
Owner:SHENZHEN AUSA PHARM CO LTD +2
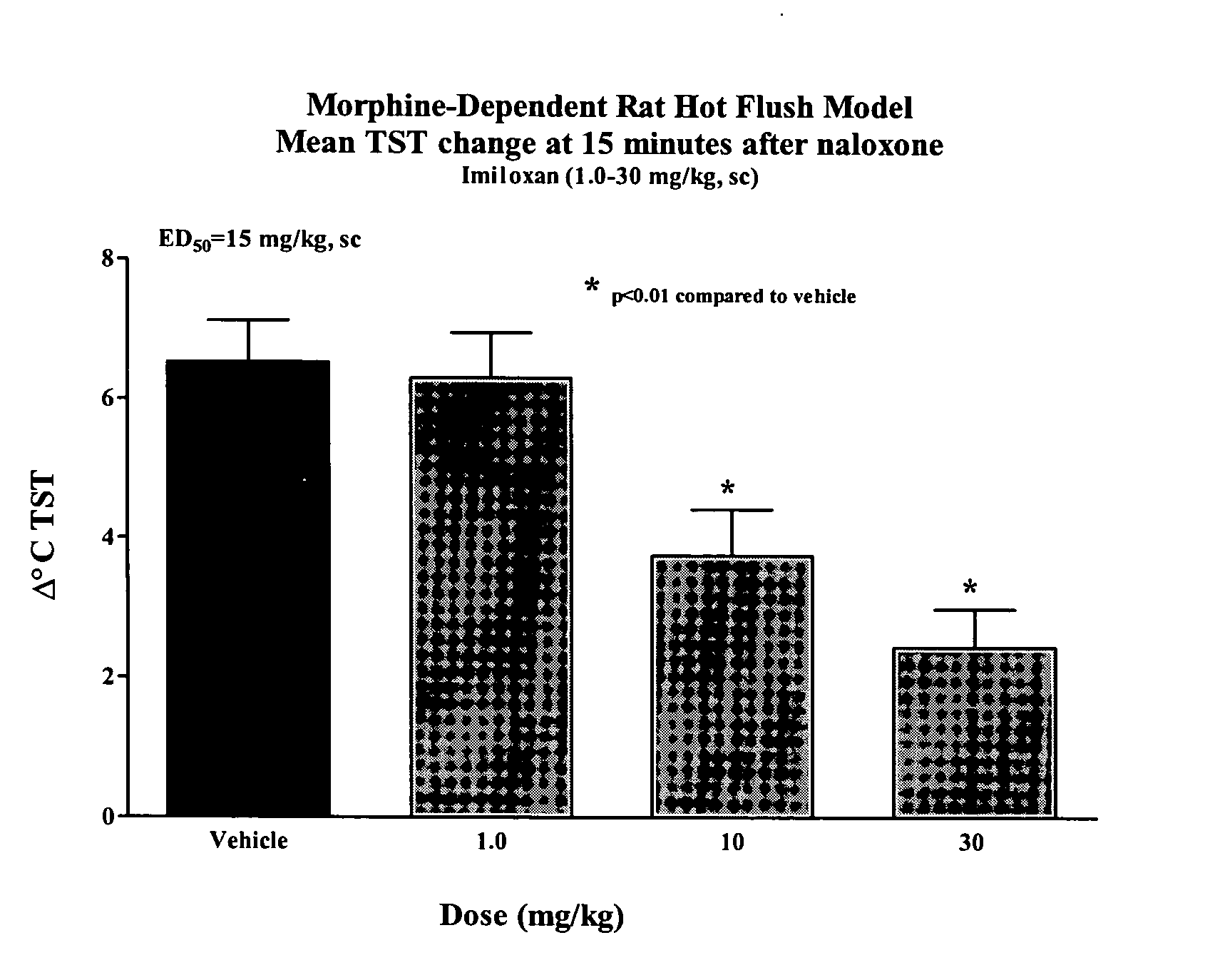
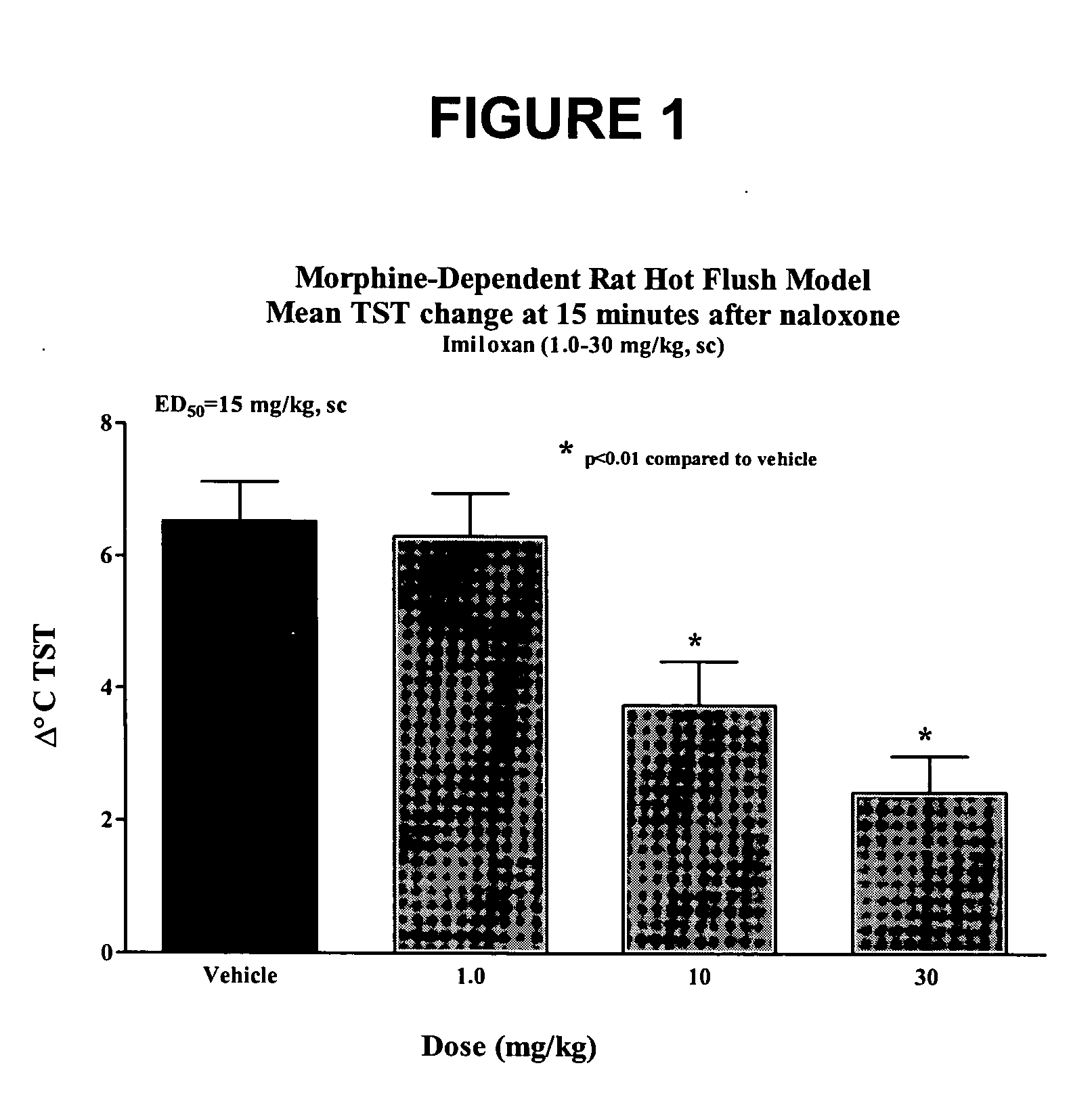
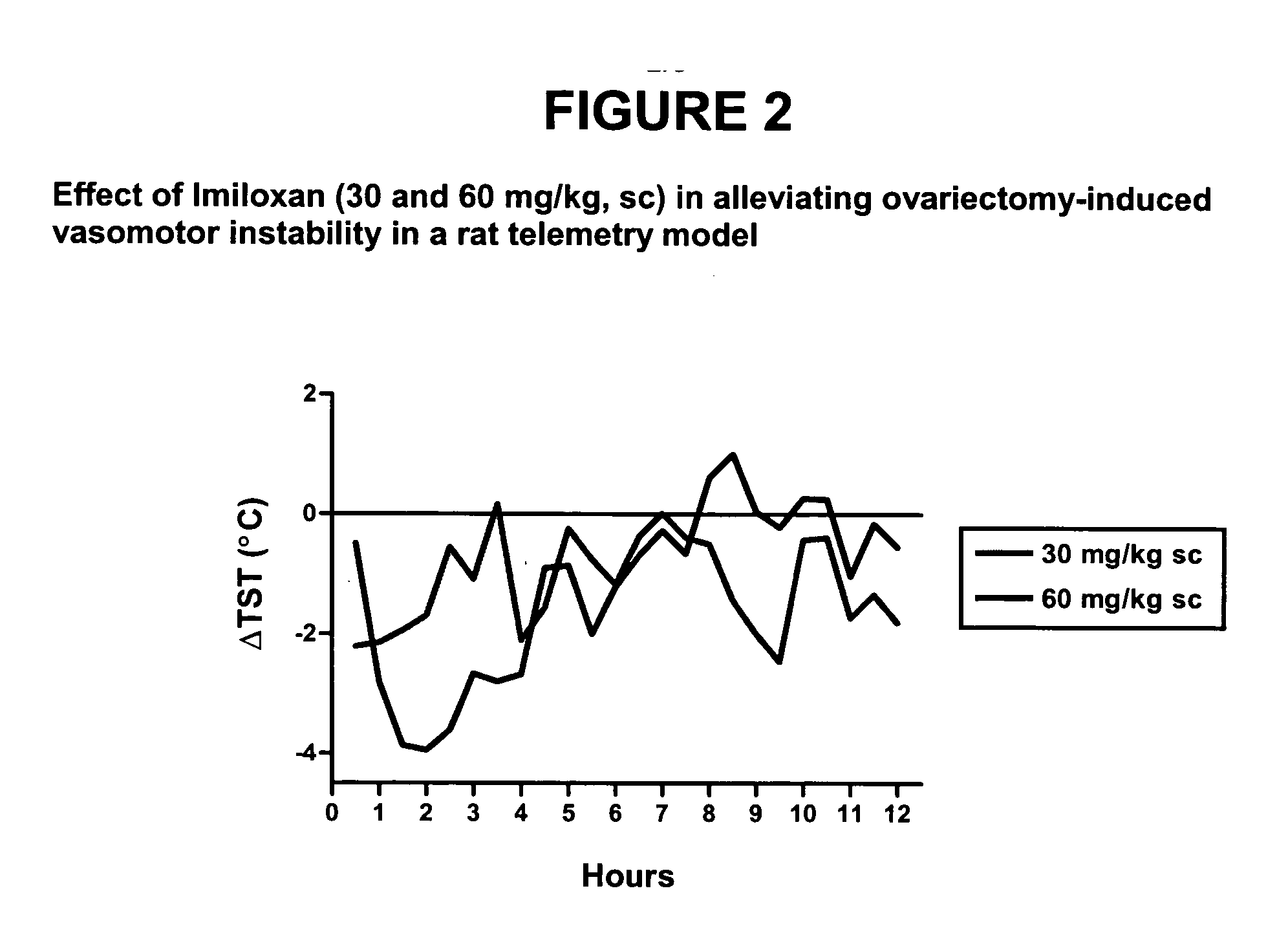
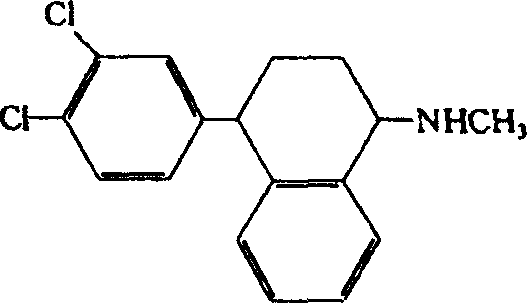
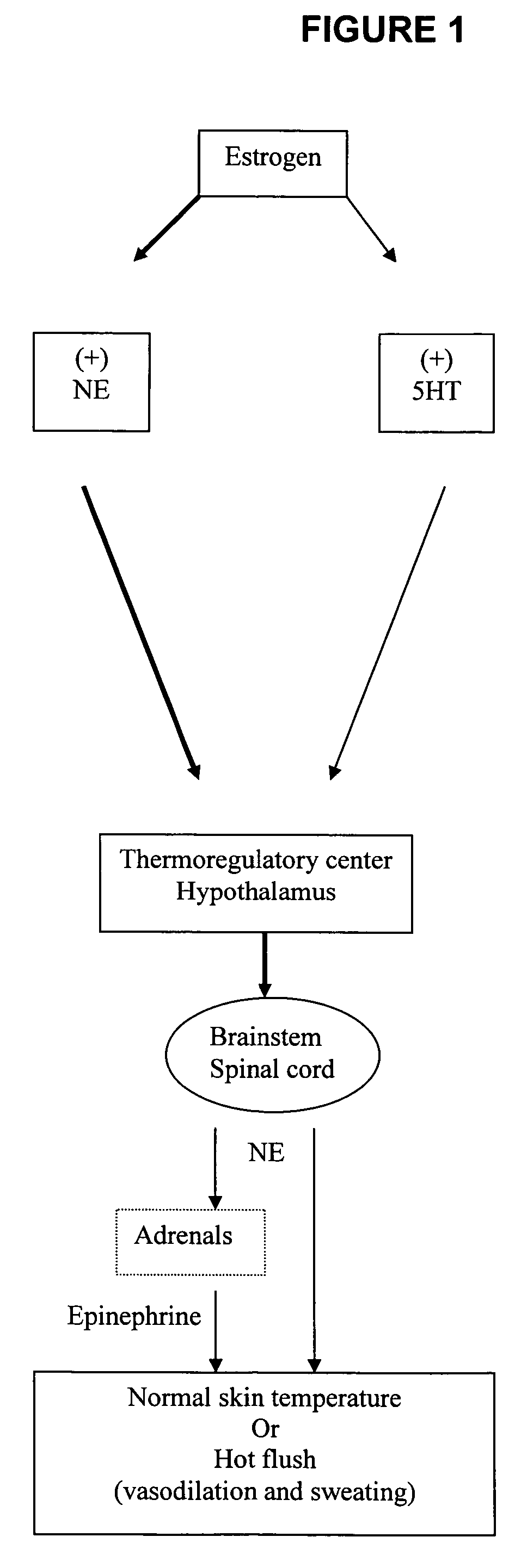
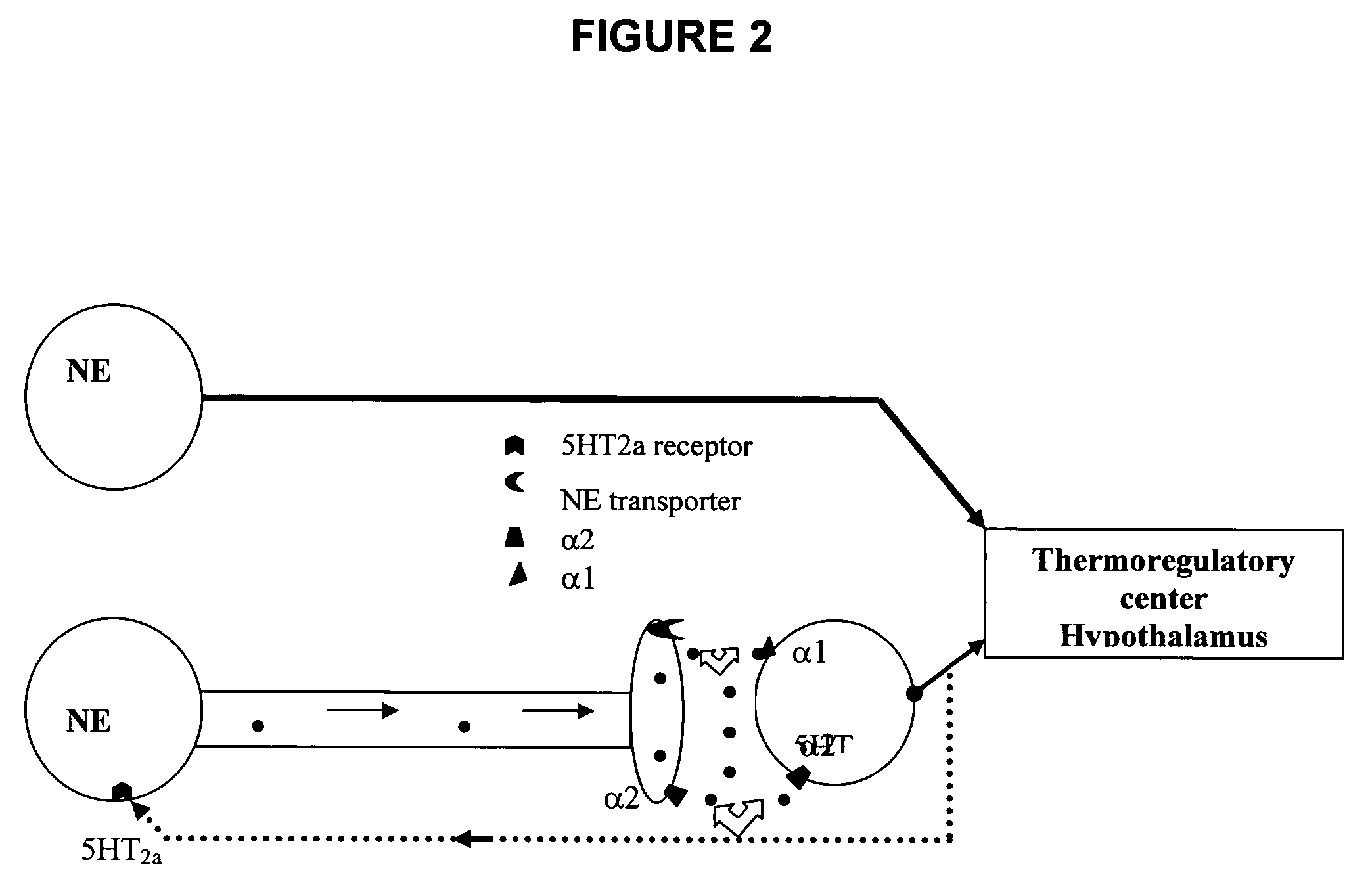
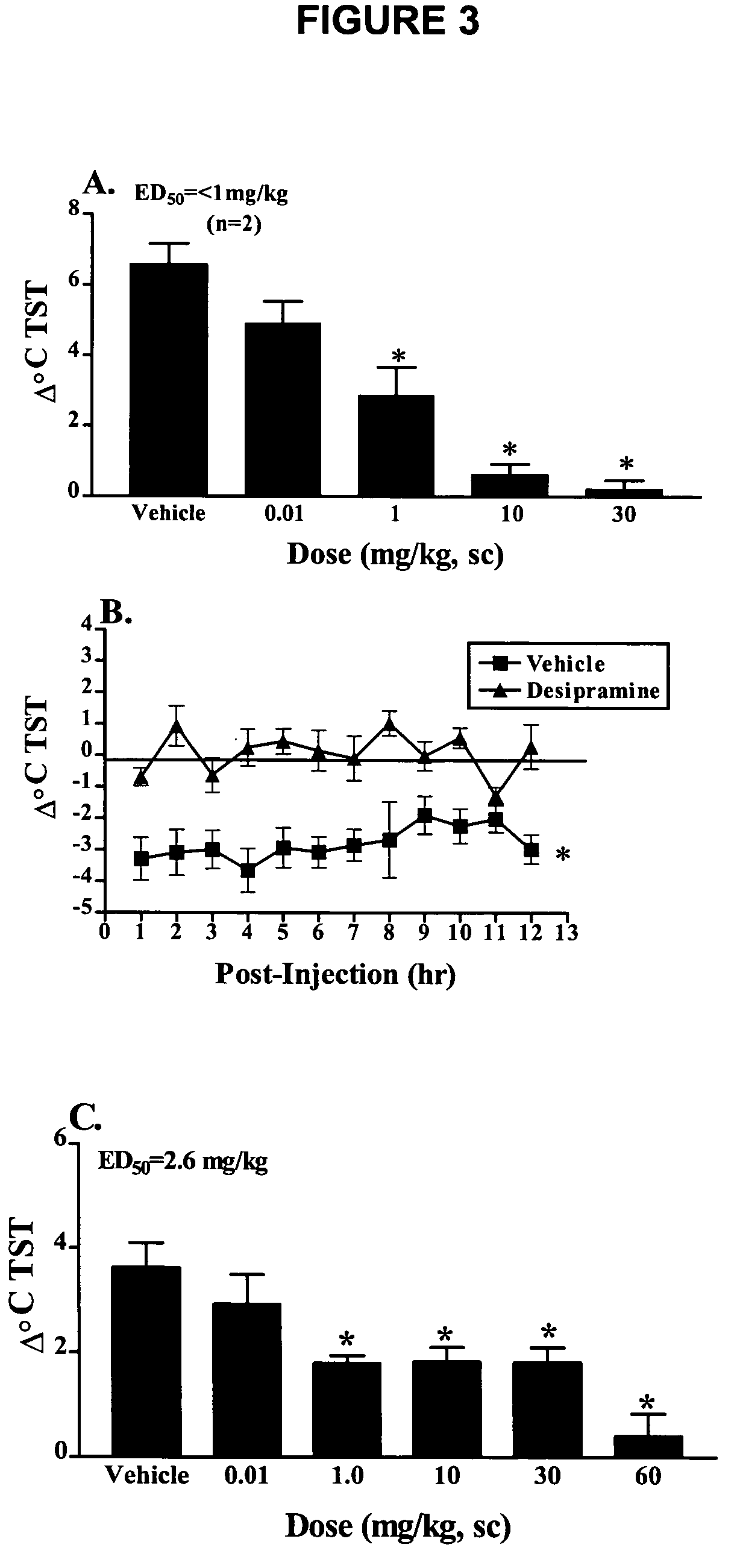
Use of norepinephrine reuptake modulators for preventing and treating vasomotor symptoms
The present invention relates to the use of compounds and composition of compounds that modulate norepinephrine levels for the prevention and treatment of vasomotor symptoms, such as hot flush, caused by, inter alia, thermoregulatory dysfunctions.
Owner:WYETH
2-(1h-indolylsulfanyl)-benzyl amine derivatives as ssri
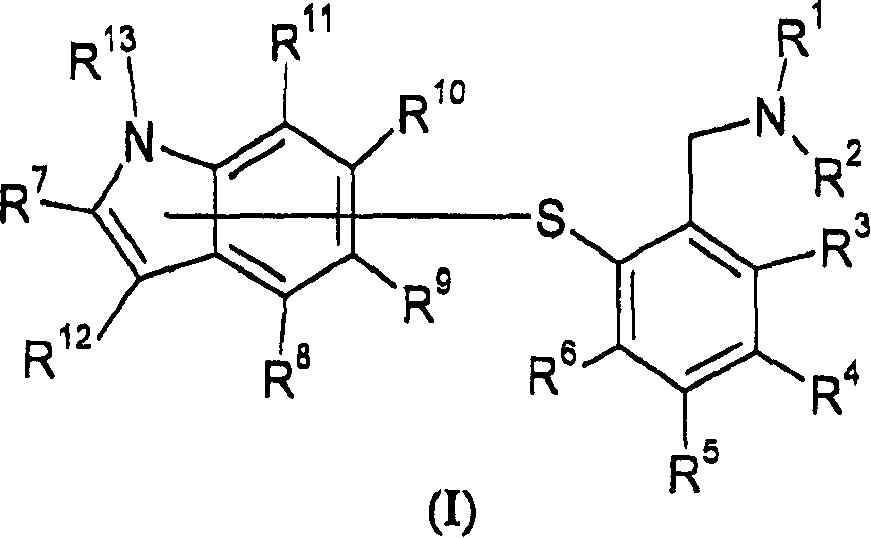
InactiveCN1898204AOrganic active ingredientsNervous disorderNorepinephrine reuptake inhibitorPain disorder
The present invention relates to aniline derivatives of the general formula I and their use as serotonin reuptake inhibitors and preferably also norepinephrine reuptake inhibitors in the treatment of depression, anxiety, affective disorders, pain disorders, attention deficit hyperactivity disorder (ADHD) and stress urinary incontinence.
Owner:H LUNDBECK AS
Low dosage combinations of fluoxetine and reboxetine for treating obesity
ActiveUS20150335649A1Reduce adverse drug reactionsReduce interactionOrganic active ingredientsNorepinephrine reuptake inhibitorBULK ACTIVE INGREDIENT
The present invention provides a pharmaceutical composition comprising a selective serotonin reuptake inhibitor (SSRI) and a norepinephrine reuptake inhibitor (NRI), particularly, fluoxetine and reboxetine, for treating obesity. Surprisingly, the inventor of the present invention discovered that use of especially low doses of the active compounds, particularly, at most 6 mg / day of reboxetine and at most 20 mg / day of fluoxetine, wherein the reboxetinerfluoxetine ratio is from about 1:4 to about 1:6, induces an effective weight loss in obese patients. Advantageously, the combinations of the present invention include very low doses of the active ingredients, thereby decreasing possible drug-drug interactions and adverse drug reaction.
Owner:BARAK NIR
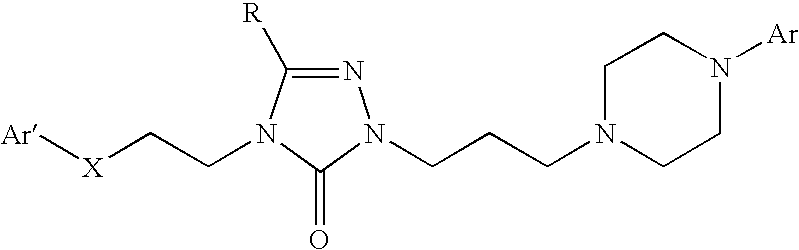
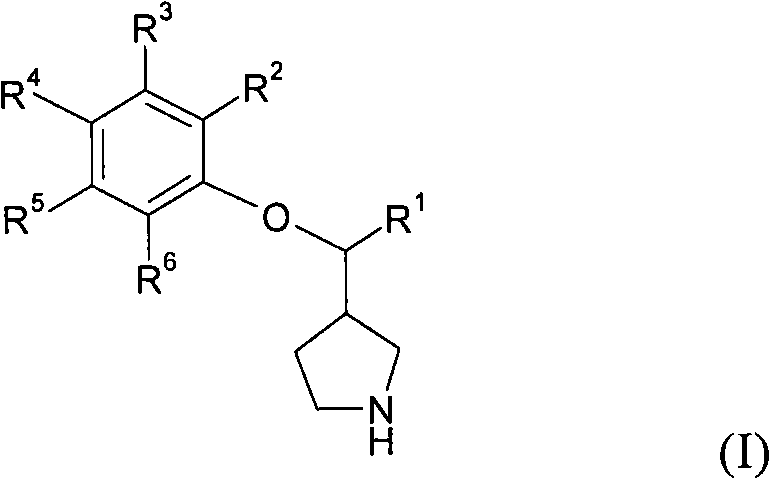
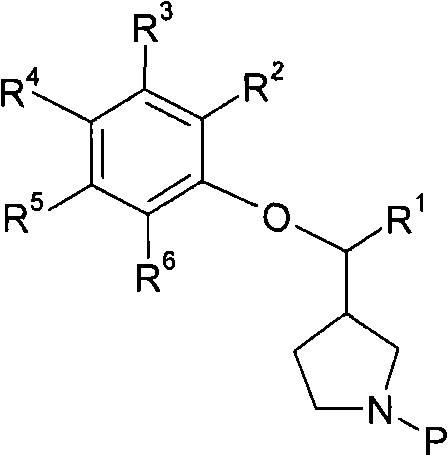
3-phenoxymethylpyrrolidine compounds
InactiveCN102471258AInhibition of reabsorptionNervous disorderOrganic chemistryNorepinephrine reuptake inhibitorAdrenergic
In one aspect, the invention relates to compounds of formula (I): where R1-6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. The compounds of formula (I) are serotonin and norepinephrine reuptake inhibitors. In another aspect, the invention relates to pharmaceutical compositions comprising such compounds; methods of using such compounds; and process and intermediates for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Treatment of disorders secondary to organic impairments
A method for treatment of neuropsychiatric symptoms or disorders emanating from primary brain or systemic impairments includes administration of an effective dose of a dopamine, serotonin, and norepinephrine reuptake inhibitor to a human in need of such treatment. The preferred reuptake inhibitor is sibutramine.
Owner:SNOWDEN PHARMA
Compounds for inhibition of 5-hydroxytryptamine and norepinephrine reuptake or for treatment of depression disorders, their preparation processes and uses thereof
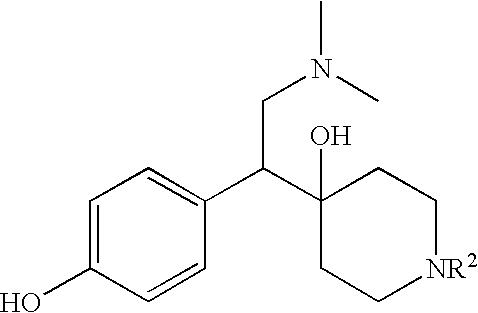
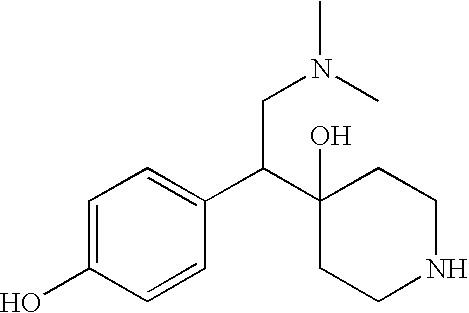
The present invention discloses compounds of formula (I), their optical isomers or pharmaceutically acceptable salts thereof, their preparation and uses thereof, wherein the definitions of R1, R2, R3 and R4 are shown in the description. These compounds are optical isomers or racemic mixtures. After these compounds are uptaken, they are metabolically transformated in vivo into 1-[2-dimethylamino-1-(4-hydroxyphenyl)-ethyl]-cyclohexanol that has neuropharmacological activity, by interrupting reuptake of 5-hydroxytryptamine (5-HT) and / or norepinephrine (NA), which is used for treating diseases associated with central nerve system, such as depression, etc.
Owner:SHANDONG LUYE PHARMA CO LTD +1
Use of norepinephrine reuptake modulators for preventing and treating vasomotor symptoms
InactiveCN1705484AAmine active ingredientsEndocrine system disorderVasomotor symptomNorepinephrine reuptake inhibitor
The present invention relates to the use of compounds and composition of compounds that modulate norepinephrine levels for the prevention and treatment of vasomotor symptoms, such as hot flush, caused by, inter alia, thermoregulatory dysfunctions.
Owner:WYETH LLC
Selective norepinephrine reuptake inhibitor and purpose thereof in preparation of medicament for attention deficit / hyperactivity disorder
InactiveCN102250053AEnhanced flipping effectHigh activityNervous disorderOrganic chemistryNorepinephrine reuptake inhibitorSide effect
The invention relates to a compound, which can be used as a selective norepinephrine reuptake inhibitor, and a purpose thereof in preparation of medicaments for ADHD (attention deficit / hyperactivity disorder). Compared with a central nervous system stimulant methylphenidate, the compound of the invention will not induce twitch or worsen dyskinesia, has little side effect, and is more suitable for ADHD patients. In addition, ADHD medicaments prepared from the compound of the invention have small application dosage.
Owner:苏州波锐生物医药科技有限公司
Serotonin-norepinephrine reuptake inhibitors (SNRIS) and sigma receptor ligands combinations
InactiveUS20160310500A1Not show side effectNot improveOrganic active ingredientsNervous disorderNorepinephrine reuptake inhibitorAdrenergic
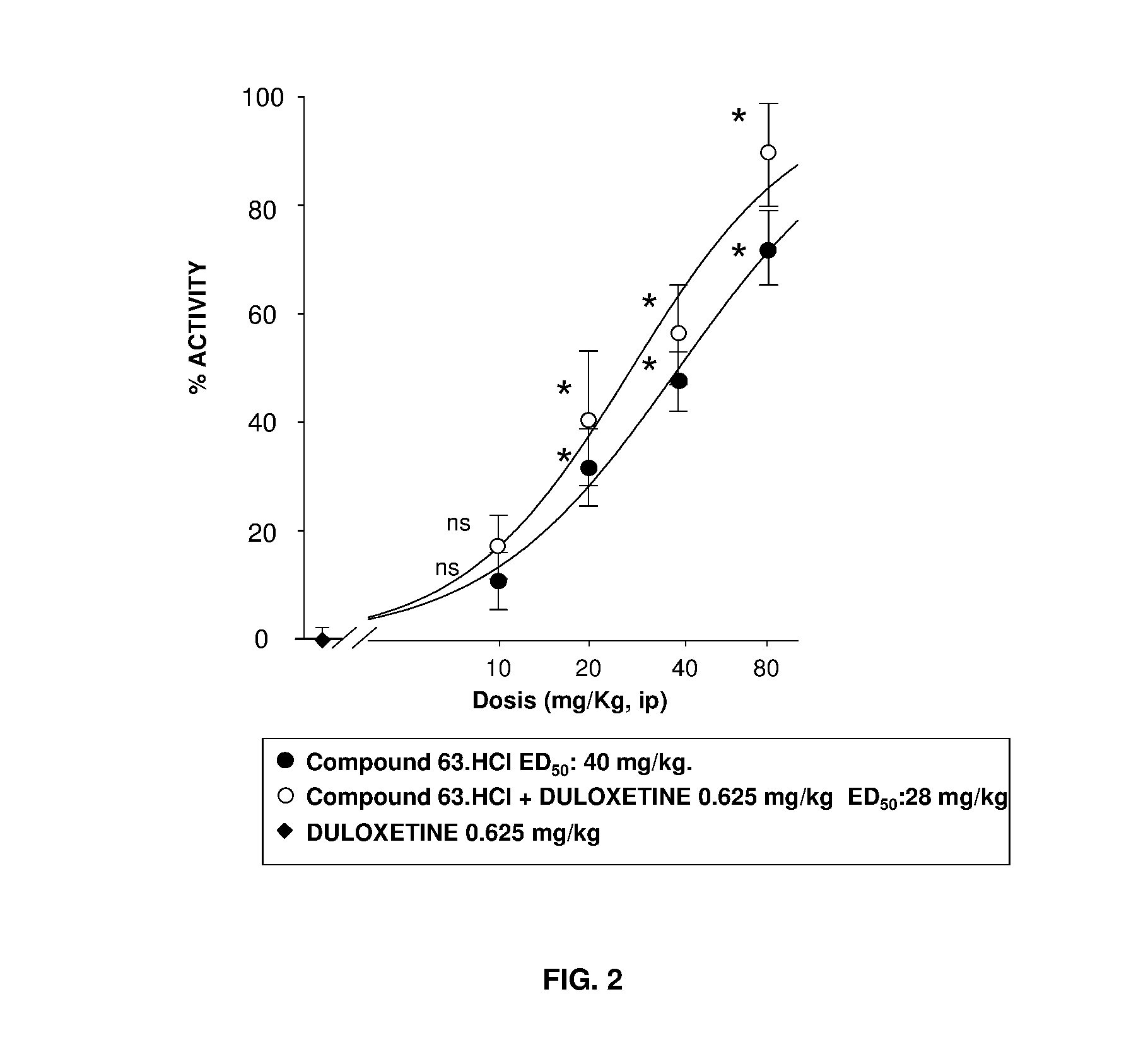
The invention refers to a synergistic combination comprising a Sigma ligand of general formula (I), and a Serotonin-Norepinephrine Reuptake Inhibitor (SNRI), a medicament comprising said active substance combination, and the use of said active substance combination for the manufacture of a medicament, particularly for the prophylaxis and / or treatment of pain.
Owner:LAB DEL DR ESTEVE SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com