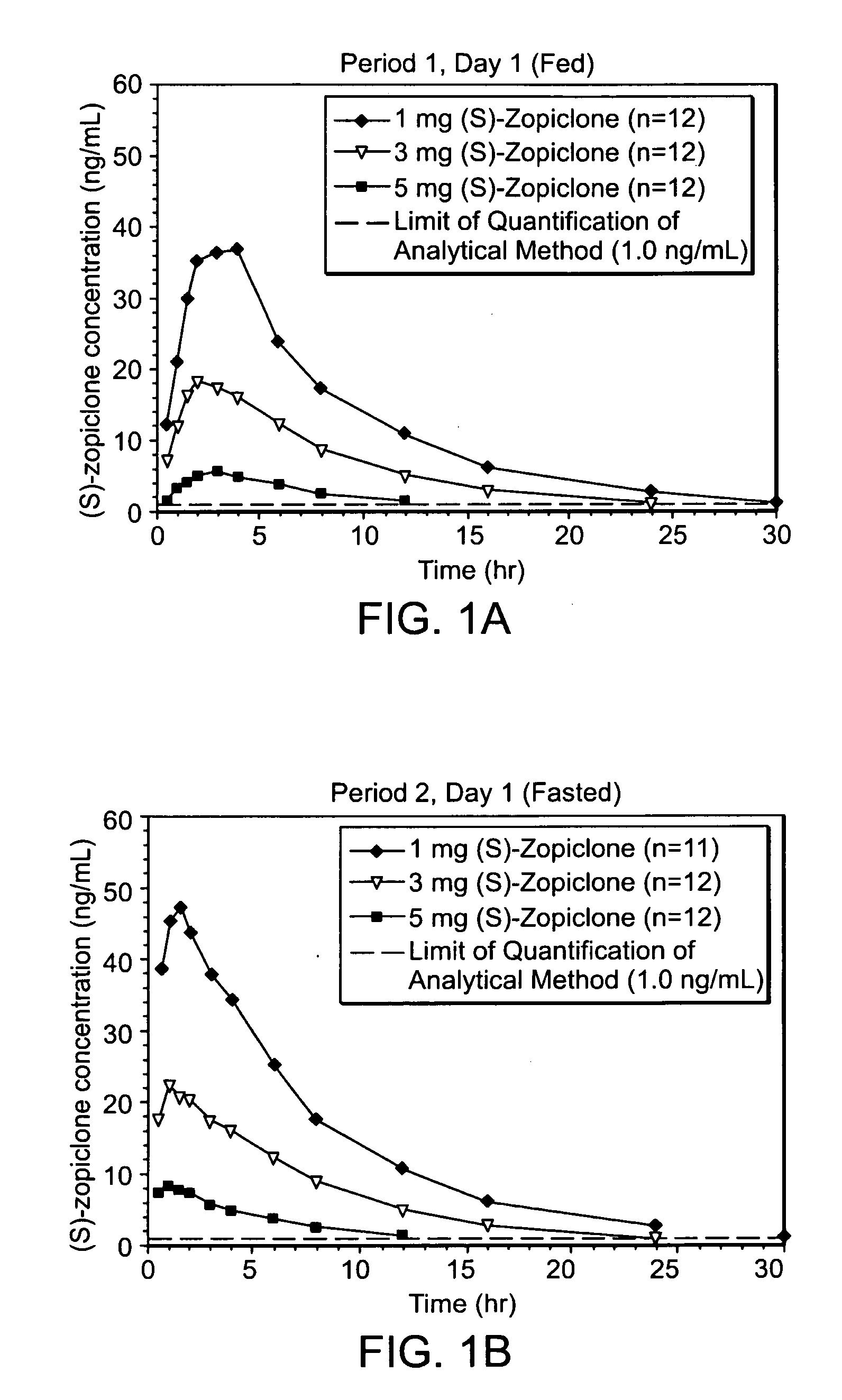
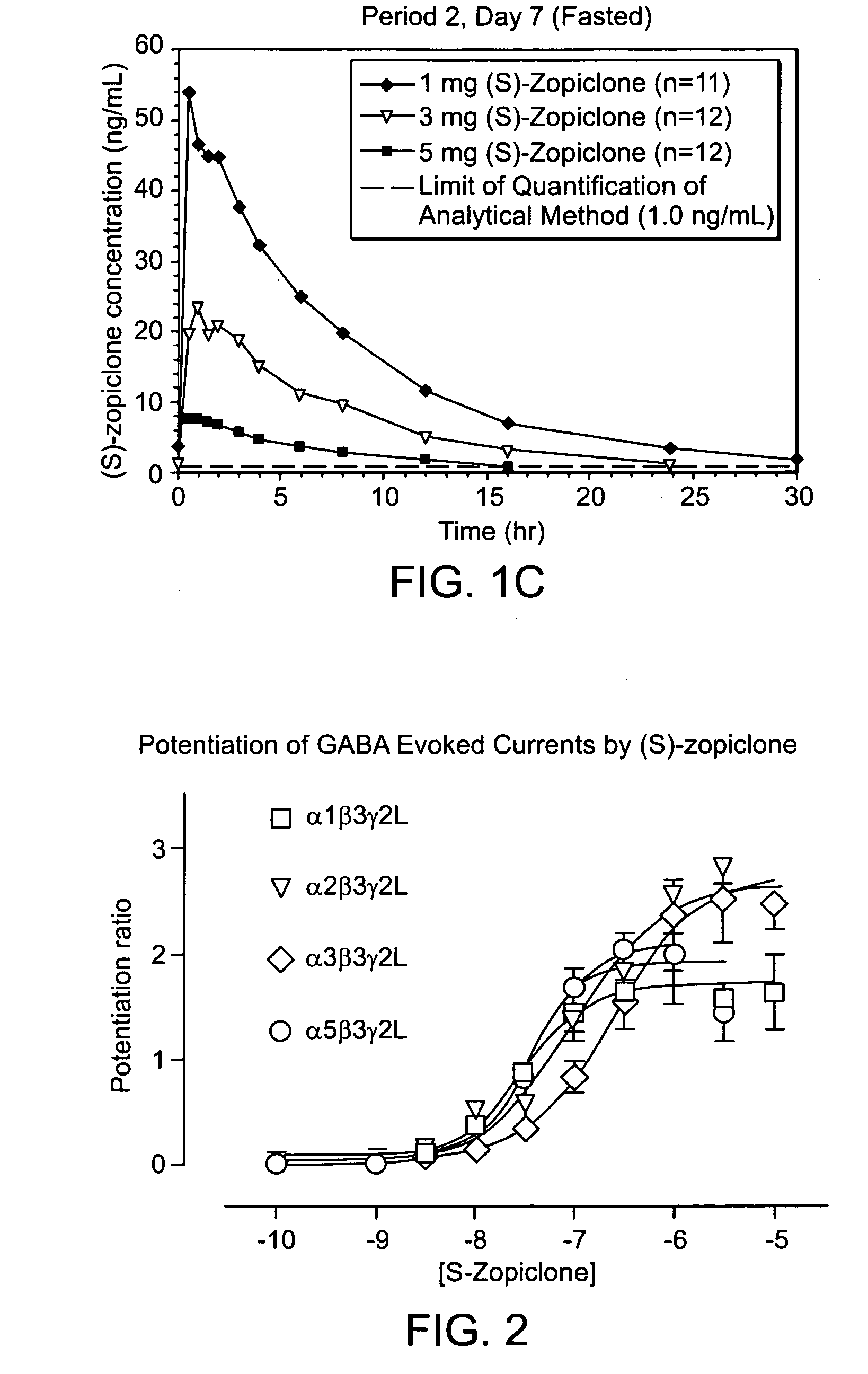
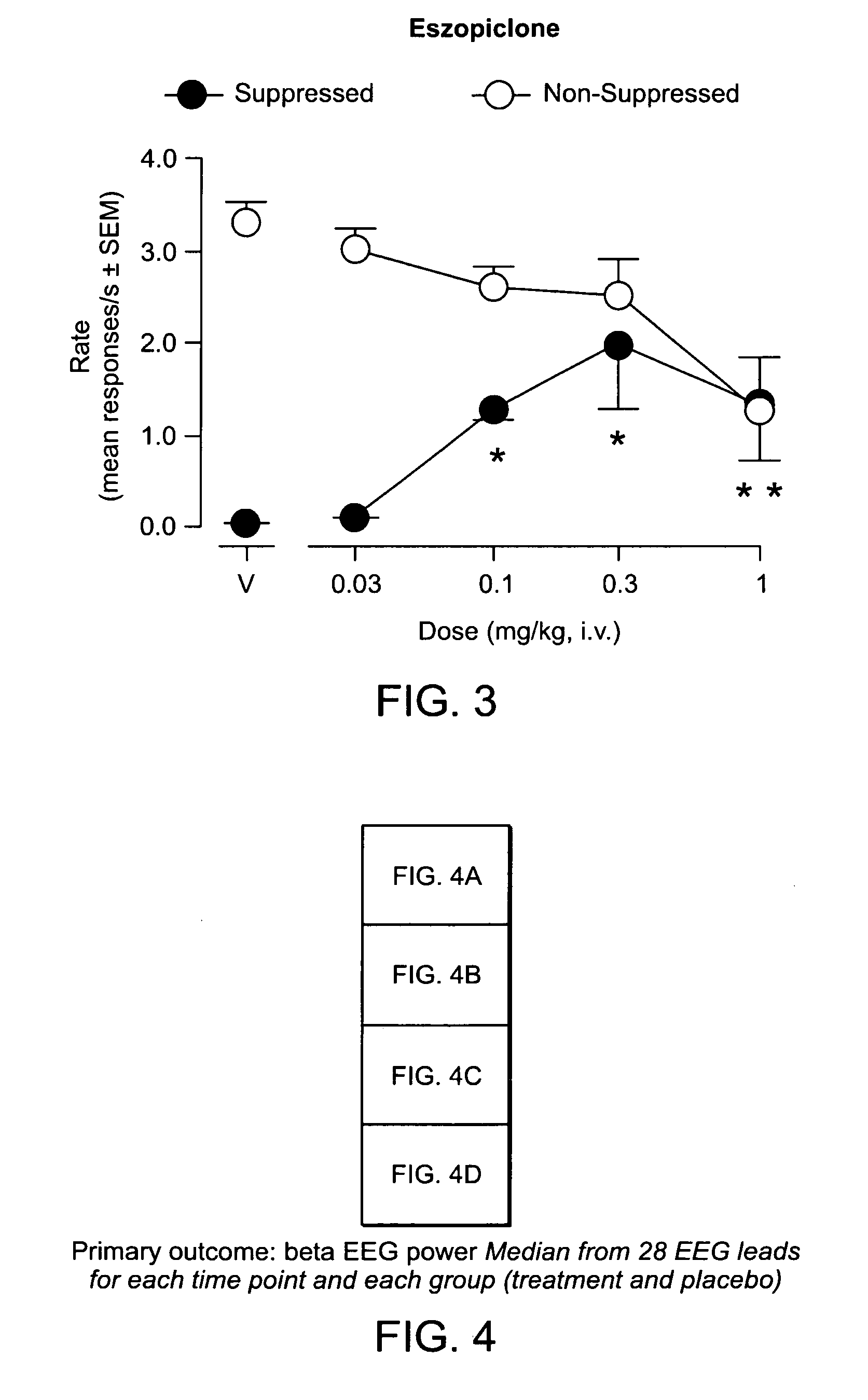
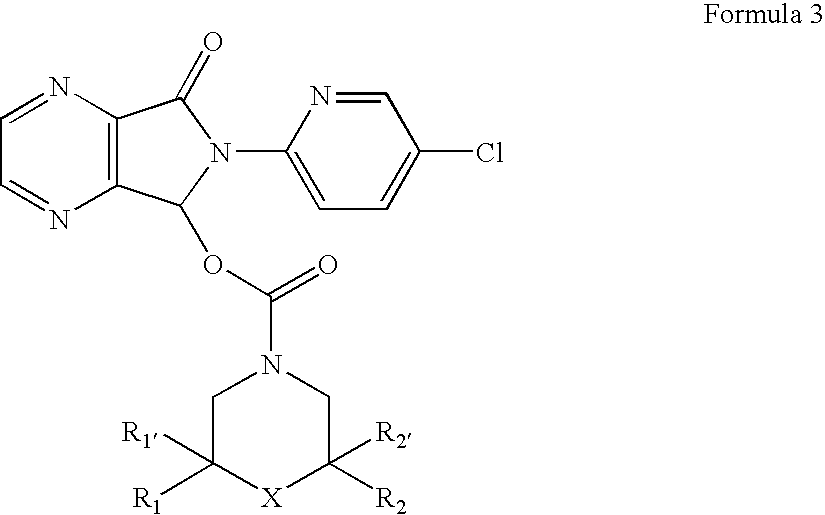
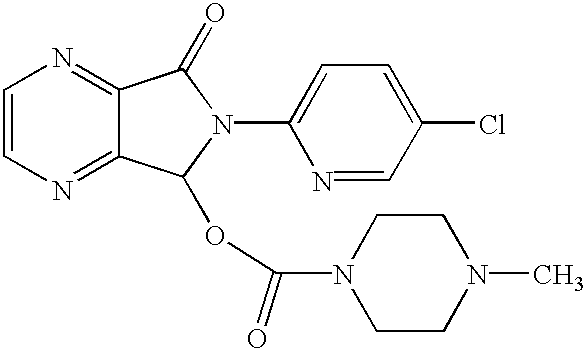
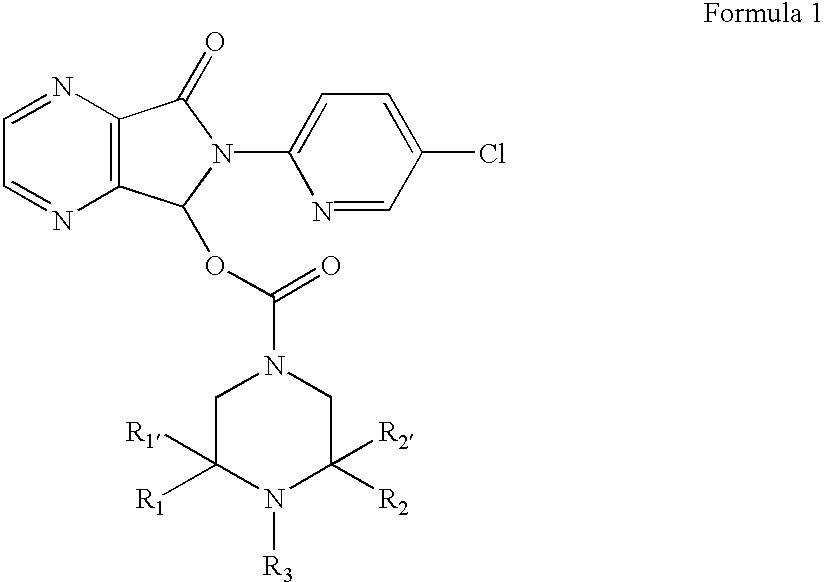
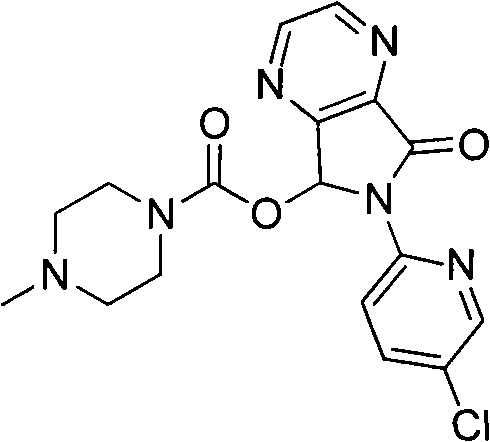
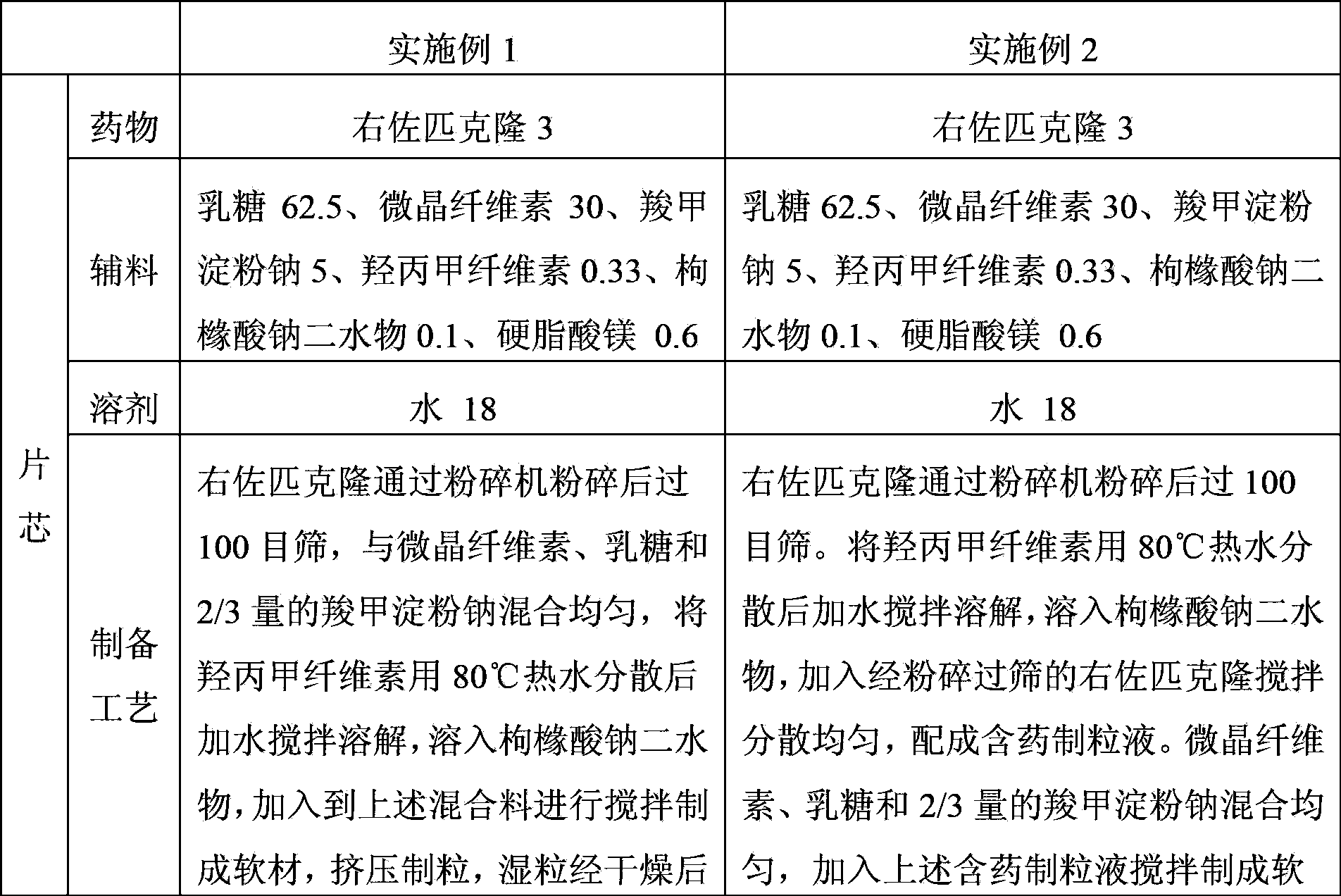
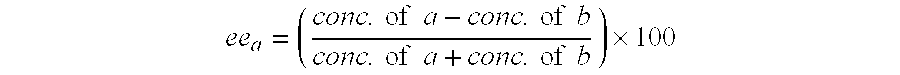Patents
Literature
77 results about "Zopiclone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zopiclone (brand names Imovane, Zimovane, and Dopareel) is a nonbenzodiazepine hypnotic agent used in the treatment of insomnia. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid in the central nervous system, via modulating benzodiazepine receptors in the same way that benzodiazepine drugs do.
Melatonin combination therapy for improving sleep quality
One aspect of the present invention relates to pharmaceutical compositions comprising a sedative agent; and melatonin or a melatonin analog, collectively referred to as “melatonin agents.” In a preferred embodiment, the sedative agent is eszopiclone. The pharmaceutical compositions of the invention are useful in the treatment of various sleep disorders. In addition, the present invention also relates to a method of treating a patient suffering from a sleep abnormality or insomnia comprising administering a therapeutically effective amount of a pharmaceutical composition of the invention.
Owner:SEPACOR INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
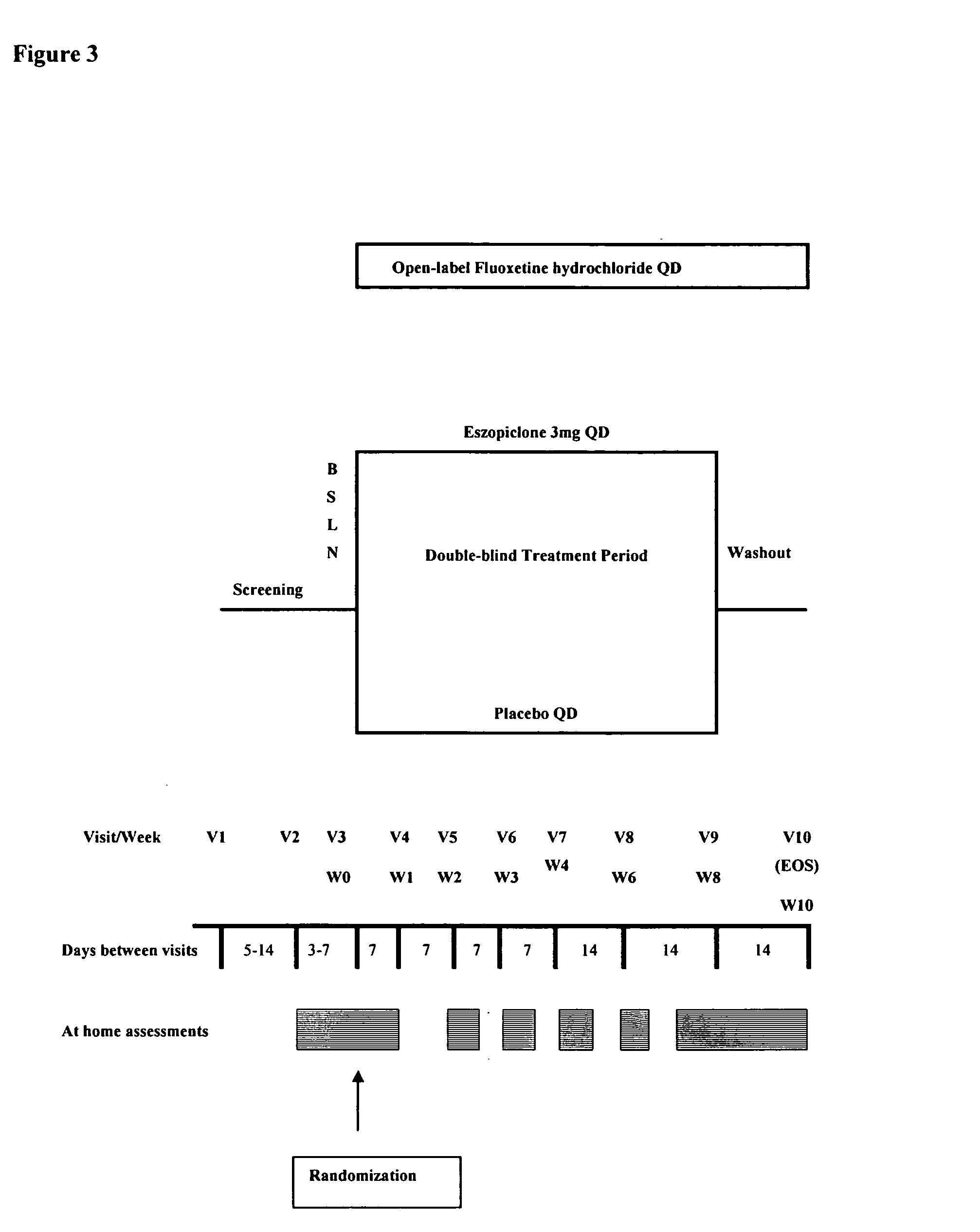
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:SEPACOR INC
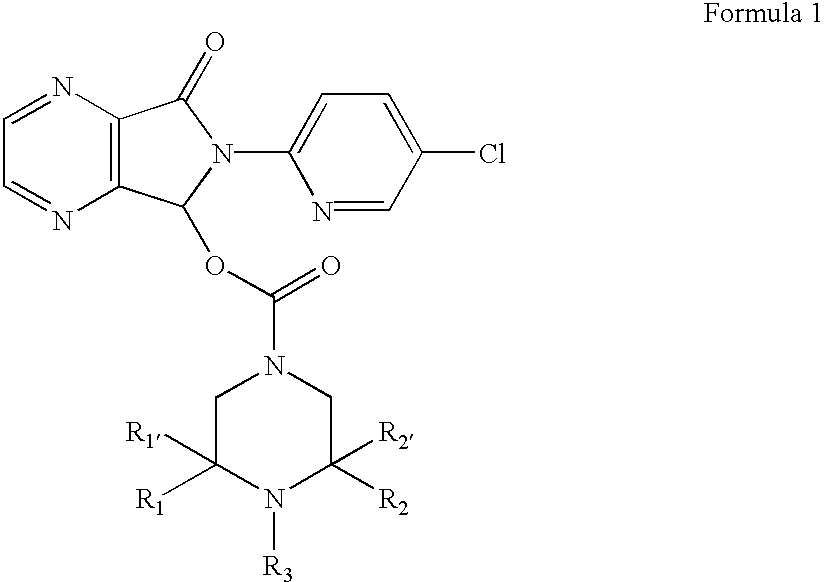
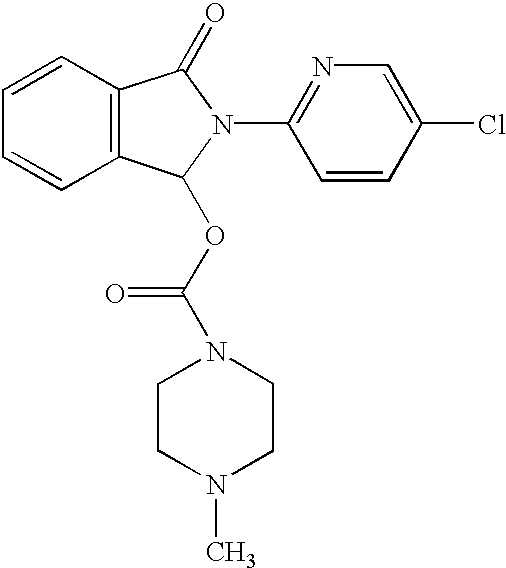
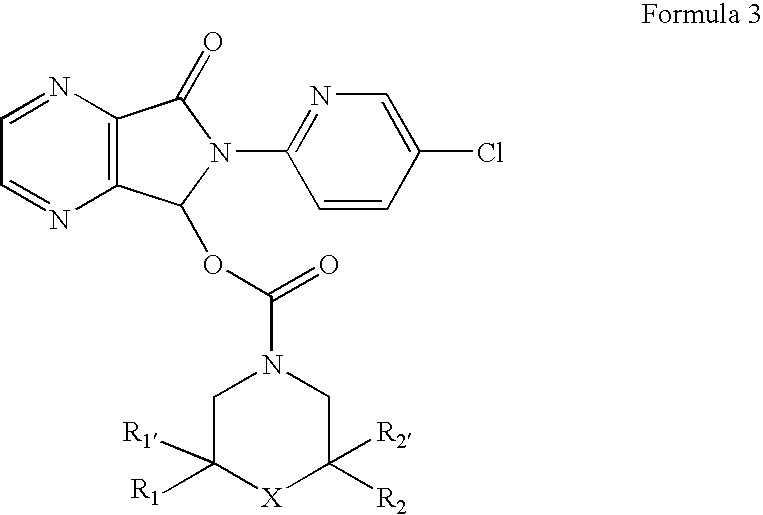
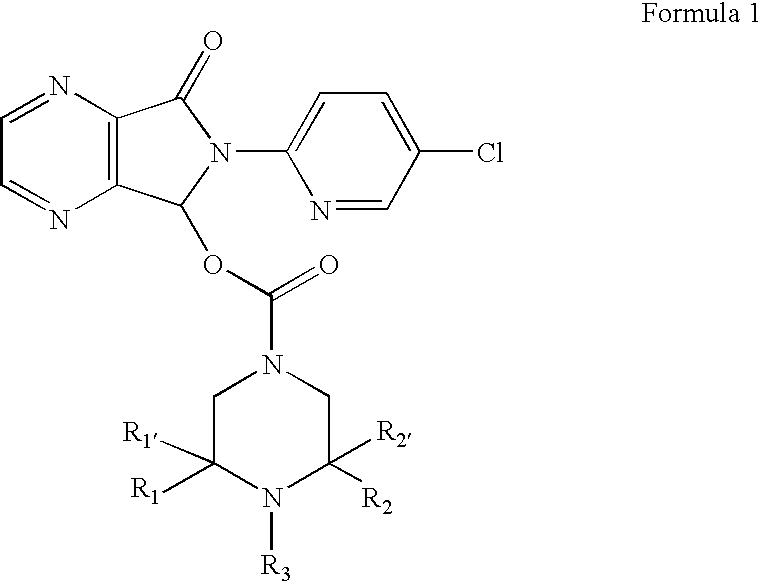
Compositions comprising zopiclone derivatives and methods of making and using the same
ActiveUS7189715B2Minimizing spread and worseningGood curative effectBiocideOrganic chemistryZopicloneMedicinal chemistry
Owner:WOODWARD SPECIALTY LLC
Methods and compositions for treating disorders, using optically pure (+) zopiclone
InactiveUS6436936B1Improve performanceReduce adverse effectsBiocideAnimal repellantsDiseaseSleeping disorders
Methods and compositions are disclosed utilizing the optically pure (+) isomer of zopiclone. This compound is a potent drug for the treatment of sleep disorders, such as insomnia, and convulsive disorders, such as epilepsy. Similarly, these novel compositions and methods are useful for the treatment of sleep disorders and convulsive disorders while avoiding the concomitant liability of adverse effects associated with the racemic mixture of zopiclone. The optically pure (+) isomer of zopiclone is also useful for treating disorders that are affected by the binding of agonists to central nervous system or peripheral benzodiazepine receptors. Also described are methods and compositions for treating disorders that are affected by binding of agonists to central nervous system or peripheral benzodiazepine receptors while avoiding the adverse effects associated with the administration of the racemic mixture of zopiclone.
Owner:SUNOVION PHARMA INC
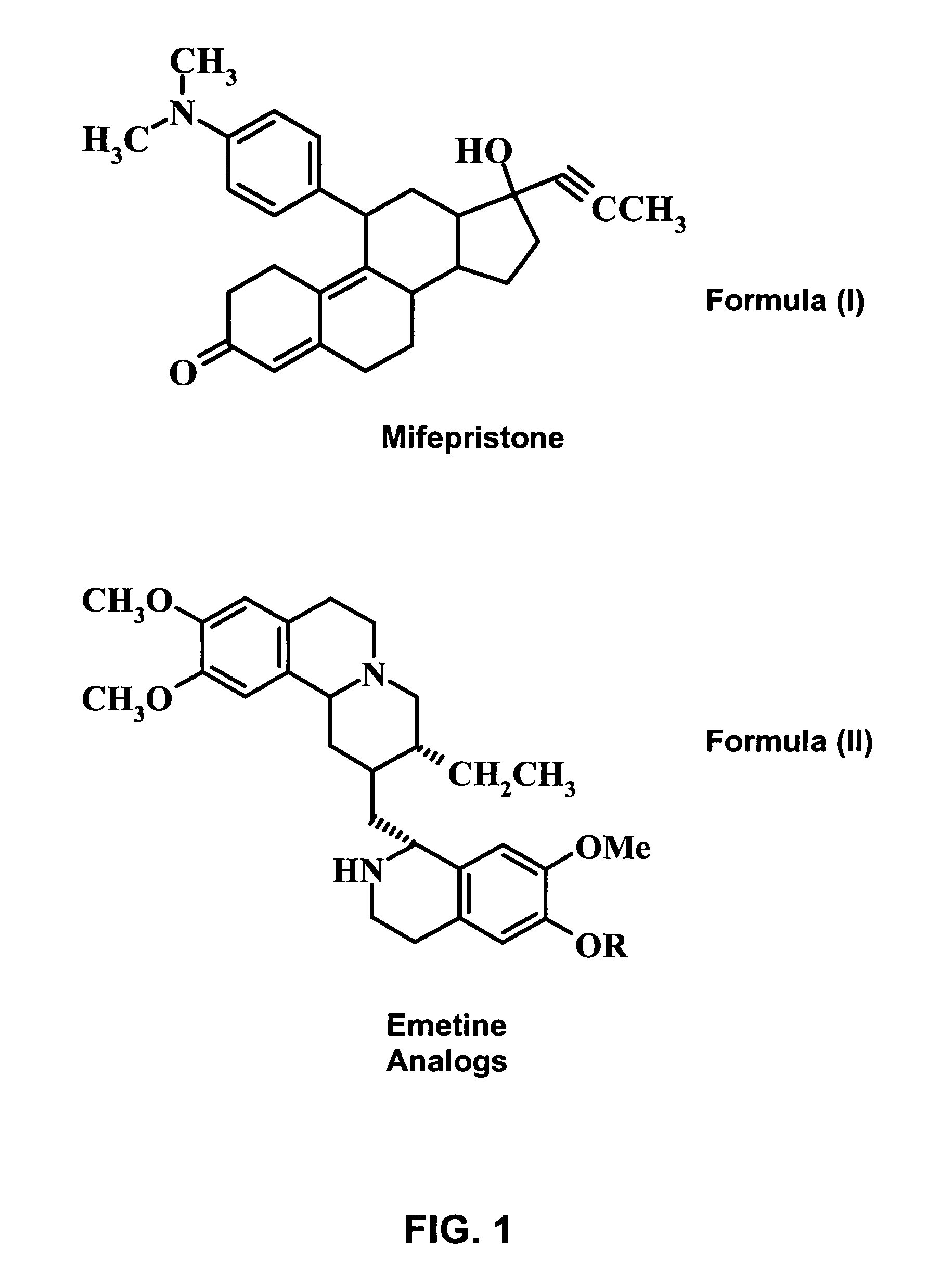
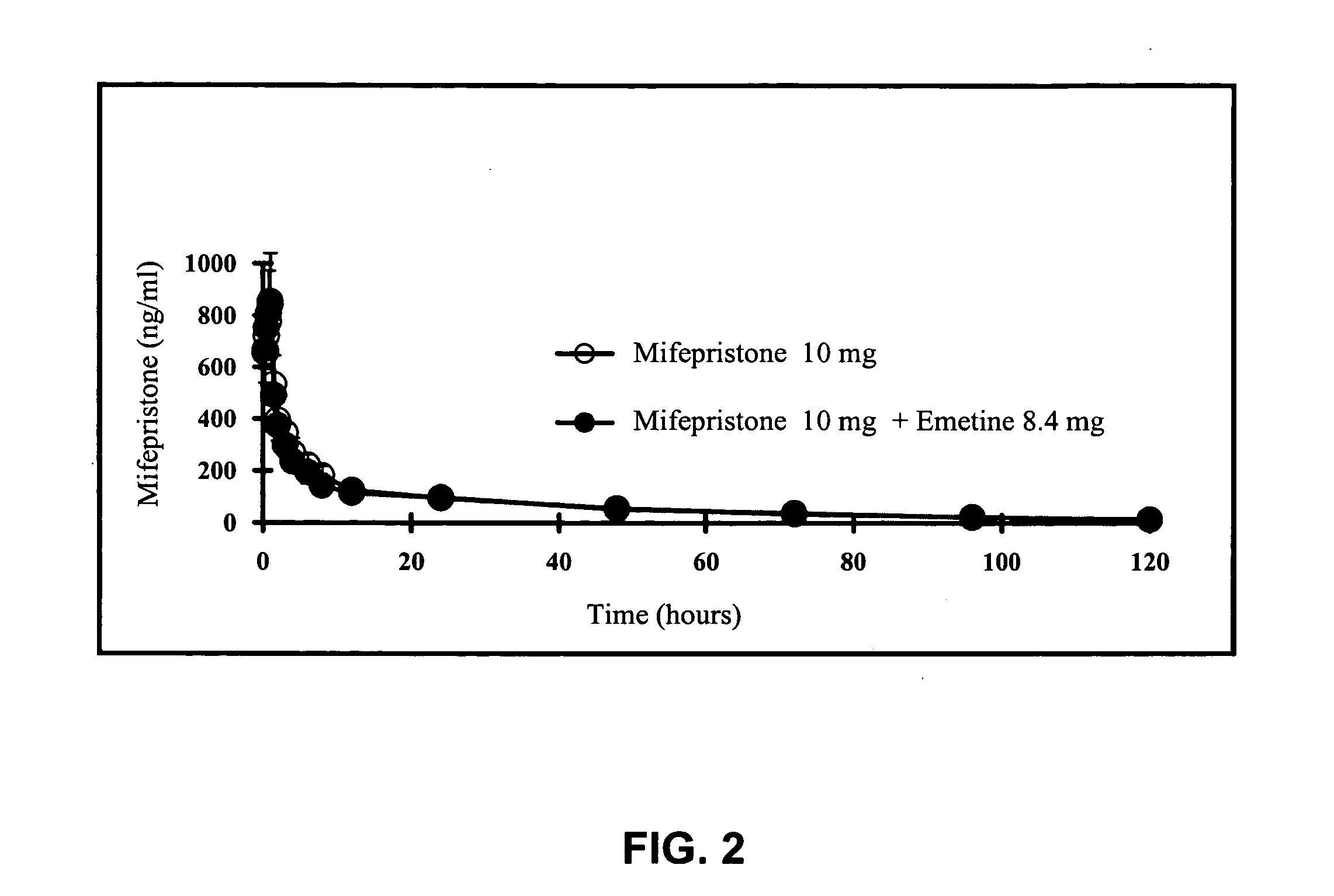
Methods and compositions for improving drug safety
A pharmaceutical composition with improved safety includes a selected amount of a vomit-inducing agent, wherein the selected amount is less than an amount needed to induce vomit in a user; and a therapeutic agent. The therapeutic agent may be selected from a sleeping pill, an anxiolytic, a hypnotic, a contraceptive agent. The therapeutic agent may also be selected from diazepam, flunitrazepam, alprazolam, triazolam, fludiazepam, midazolam, estazolam, zopiclone, and a combination thereof. The vomit-inducing agent may be selected from emetine, cephaeline, and a combination thereof.
Owner:LOTUS PHARMA CO LTD
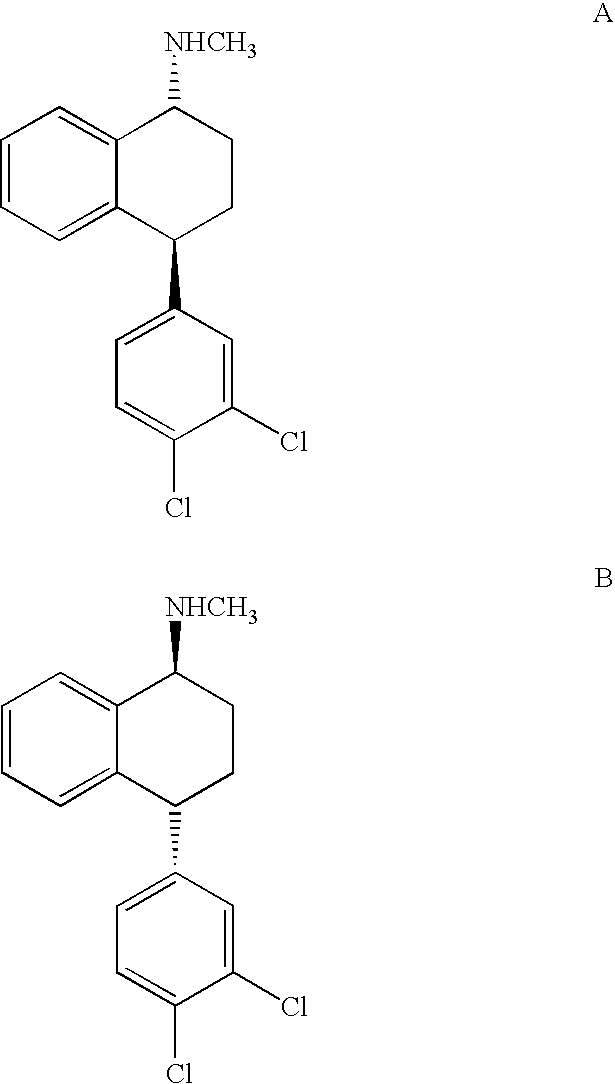
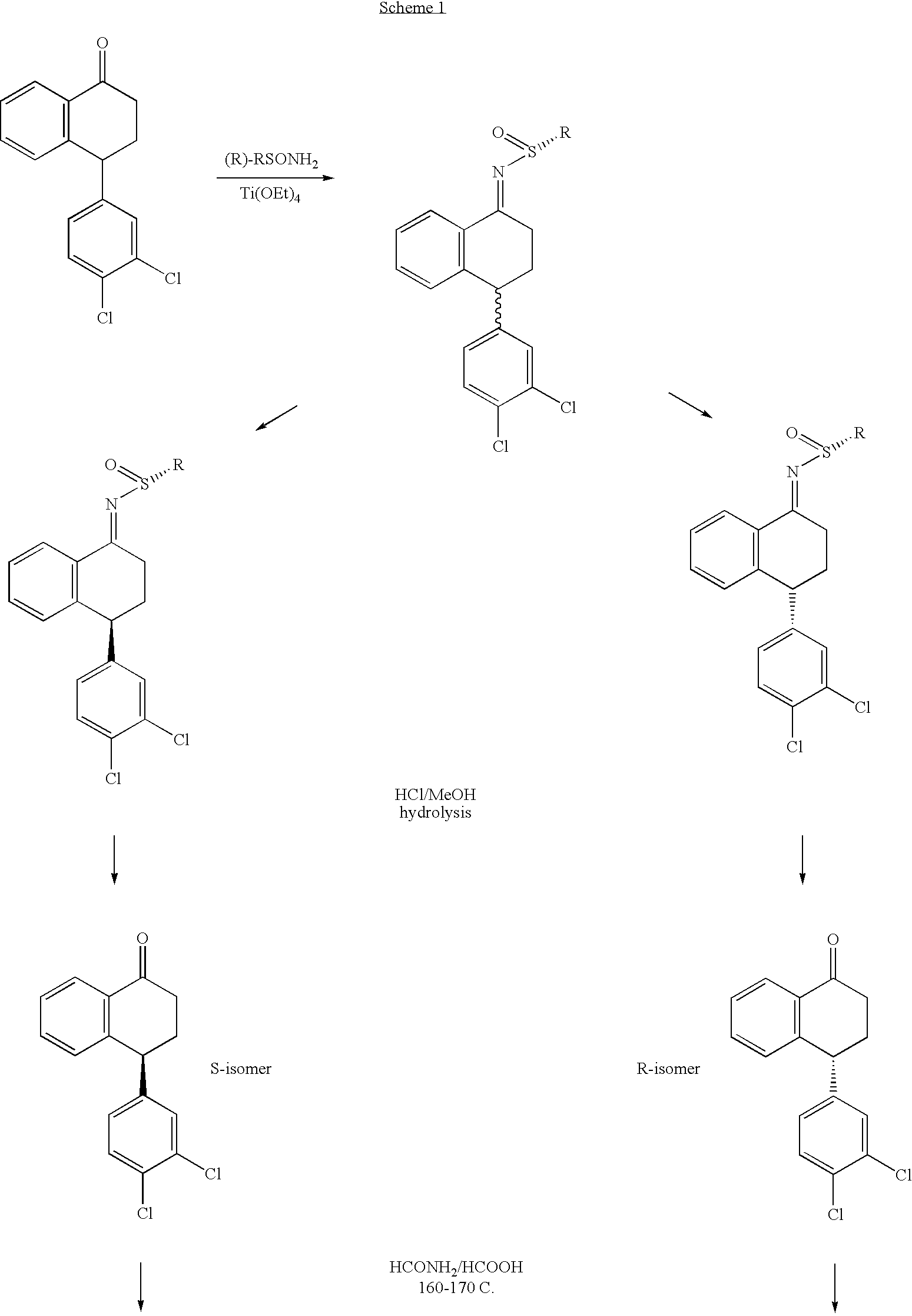
Combinations of Eszopiclone and Trans 4-(3,4-Dichlorophenyl)-1,2,3,4-Tetrahydro-N-Methyl-1-Napthalenamine or Trans 4-(3,4-Dichlorophenyl)-1,2,3,4-Tetrahydro-1-Napthalenamine, and Methods of Treatment of Menopause and Mood, Anxiety, and Cognitive Disorders
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is trans 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-napthalenamine or trans 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-napthalenamine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Combinations of Eszopiclone and an Antidepressant
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is an antidepressant. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Treatment of anxiety with eszopiclone
InactiveUS20080175903A1Improved liabilityImprove securityBiocideNervous disorderANXIETY COMPLEXAnxiety
The present disclosure provides a unit dosage form with an anxiolytic dosage of zopiclone particularly eszopiclone. Also provided is a method for treatment or prophylaxis of anxiety using a subsedative dosage of zopiclone particularly eszopiclone.
Owner:SEPACOR INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:WOODWARD SPECIALTY LLC
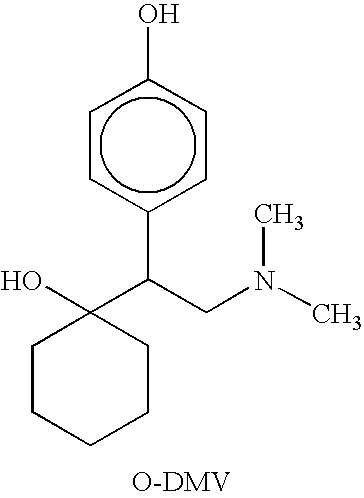
Combinations of Eszopiclone and O-Desmethylvenlafaxine, and Methods of Treatment of Menopause and Mood, Anxiety, and Cognitive Disorders
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is O-desmethylvenlafaxine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
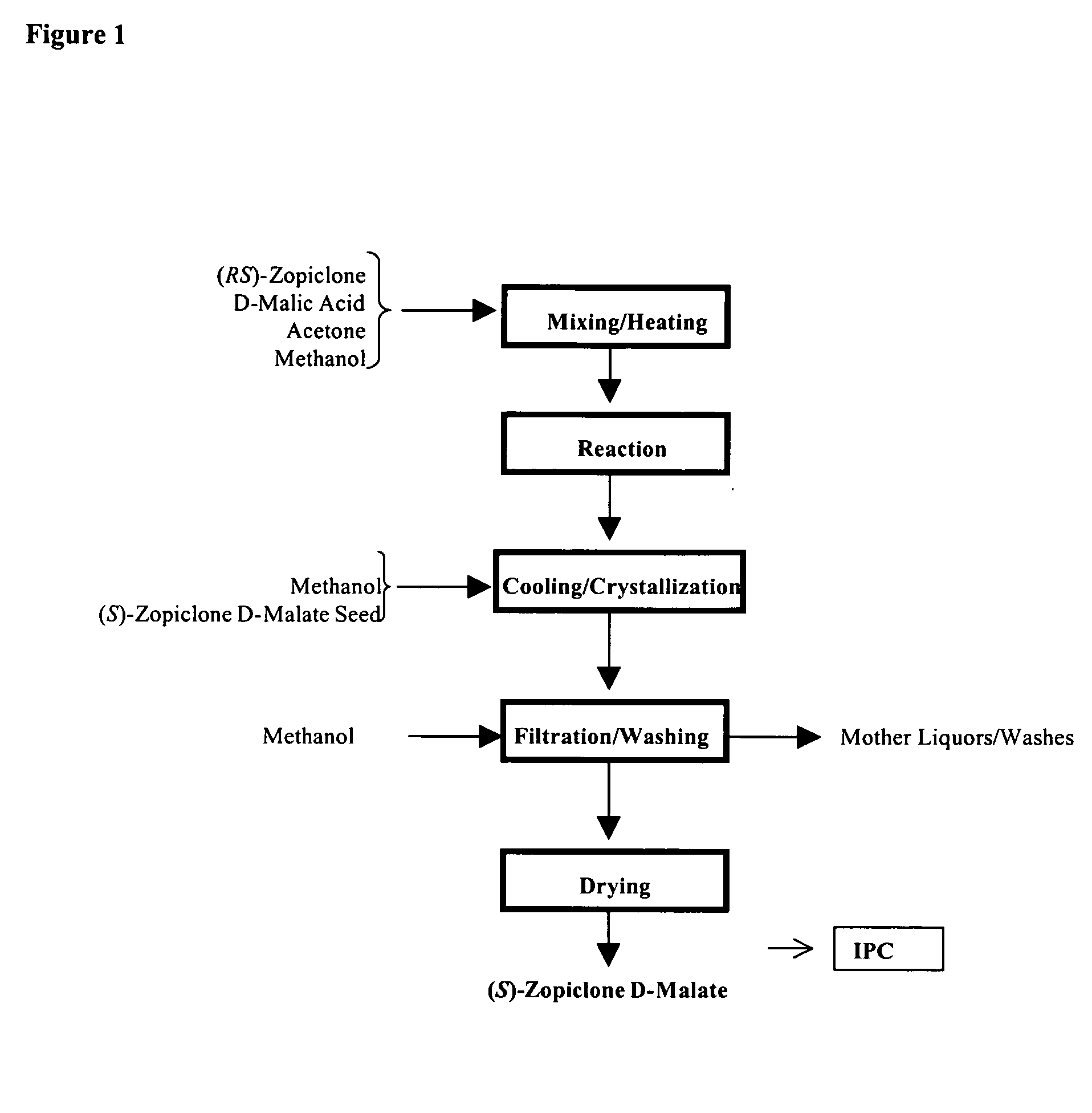
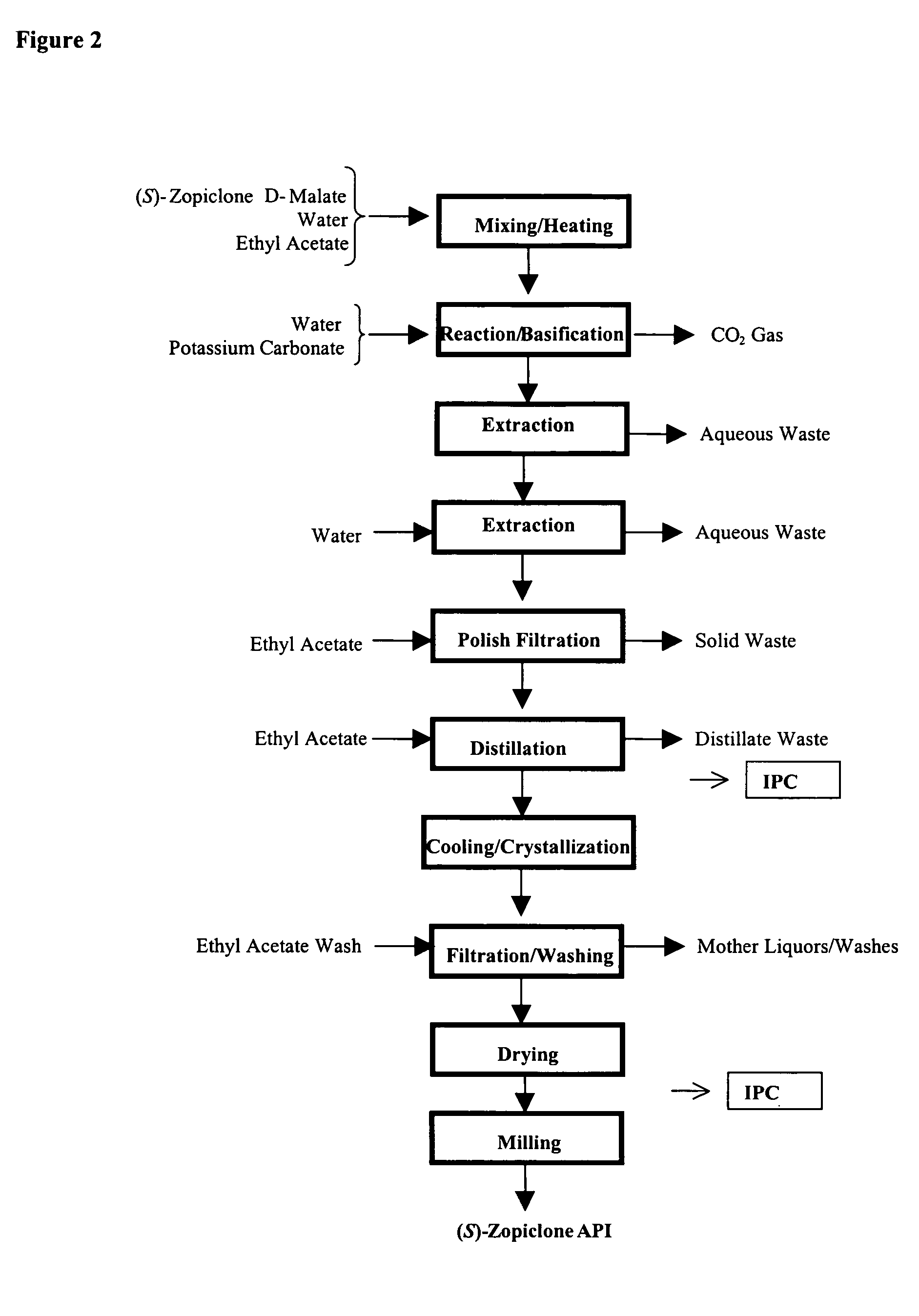
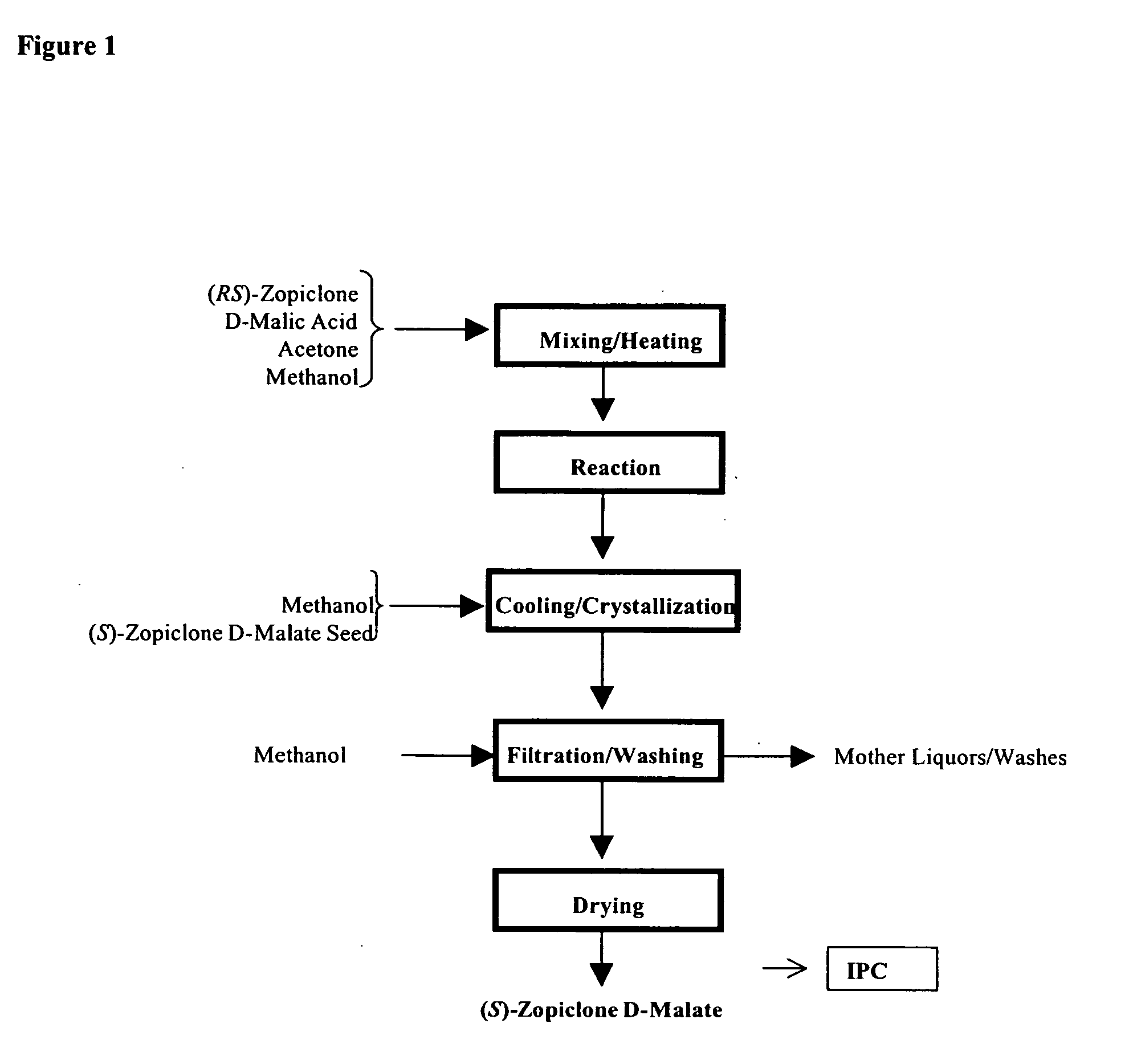
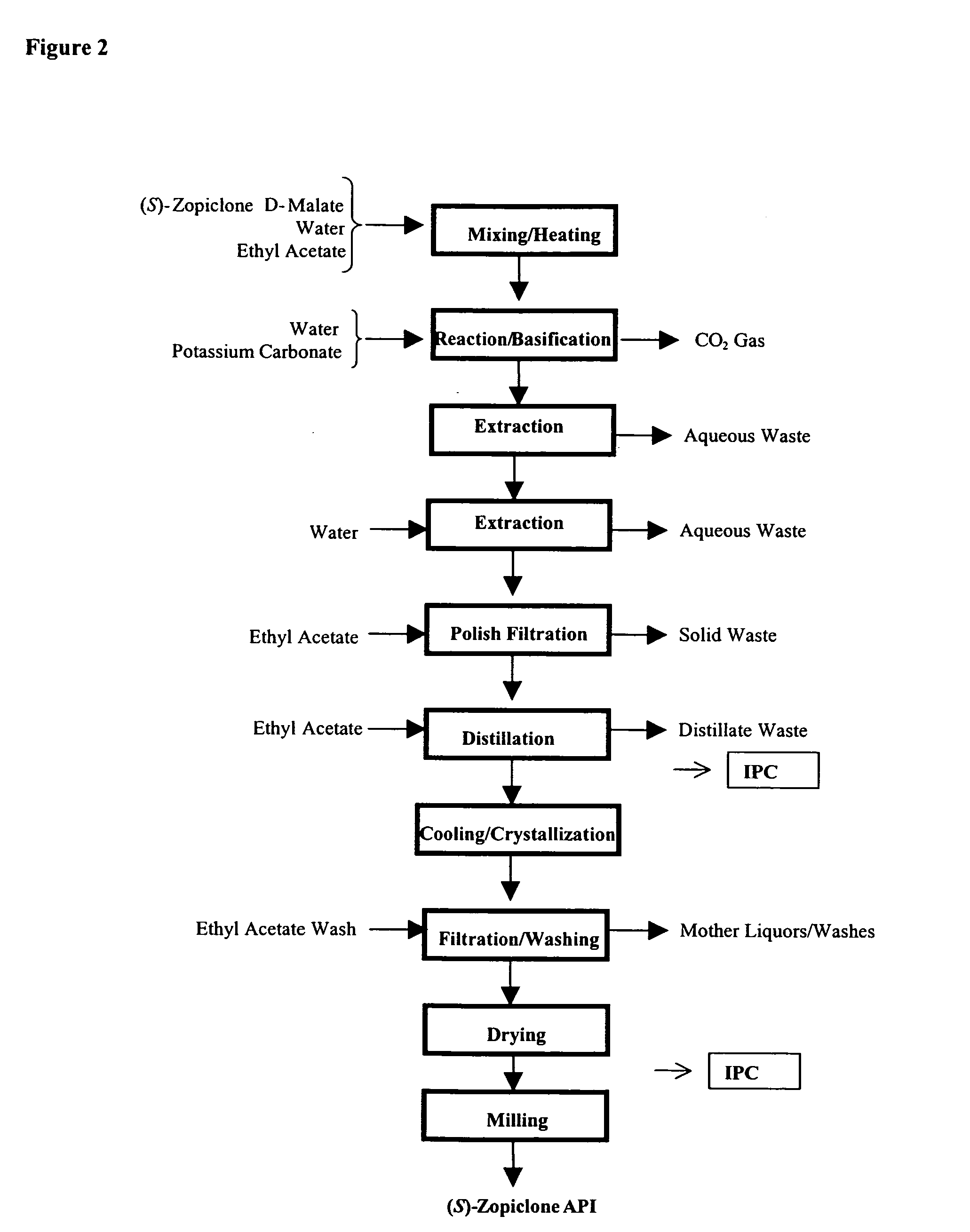
Method for resoluting zopiclone
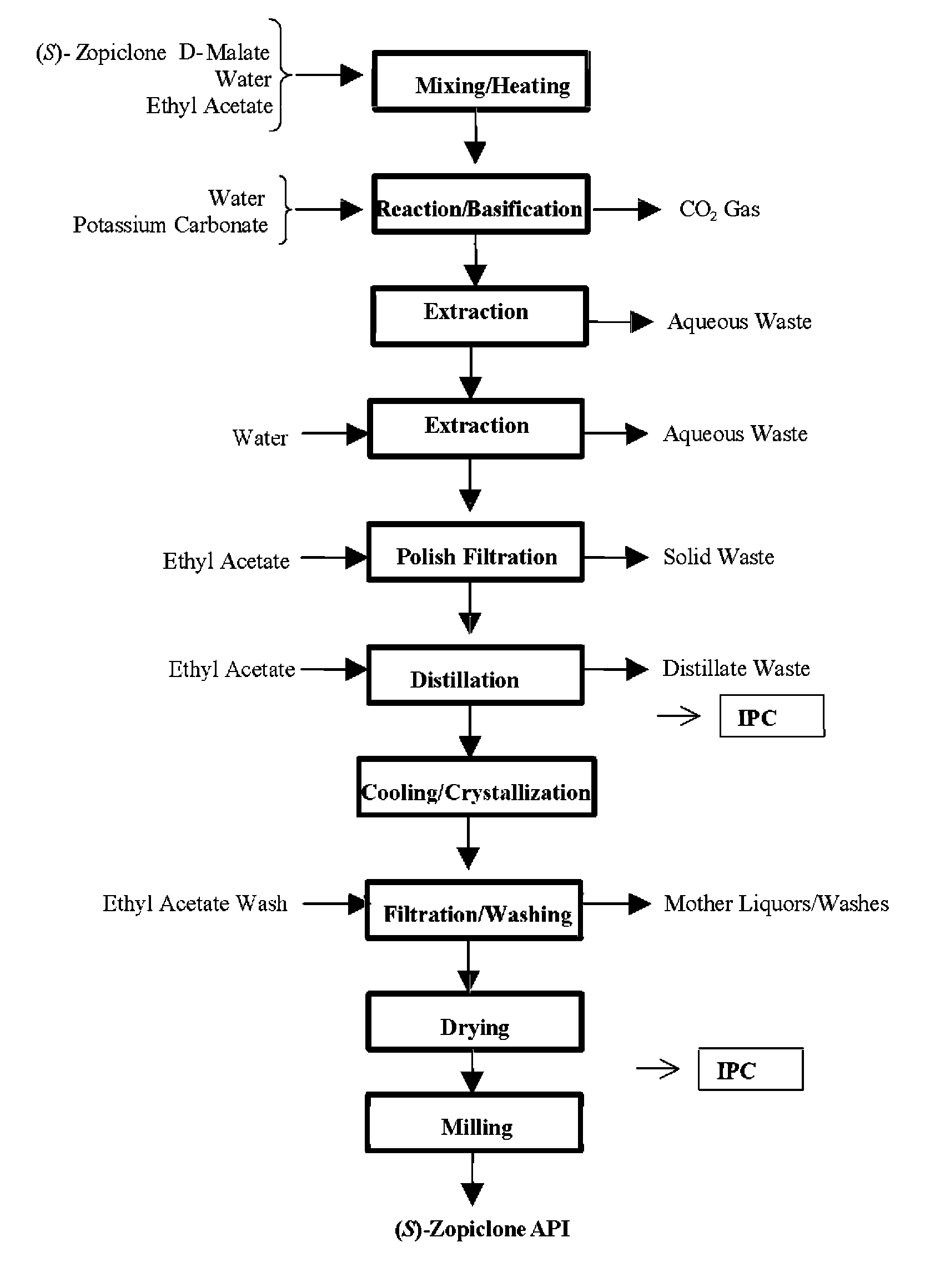
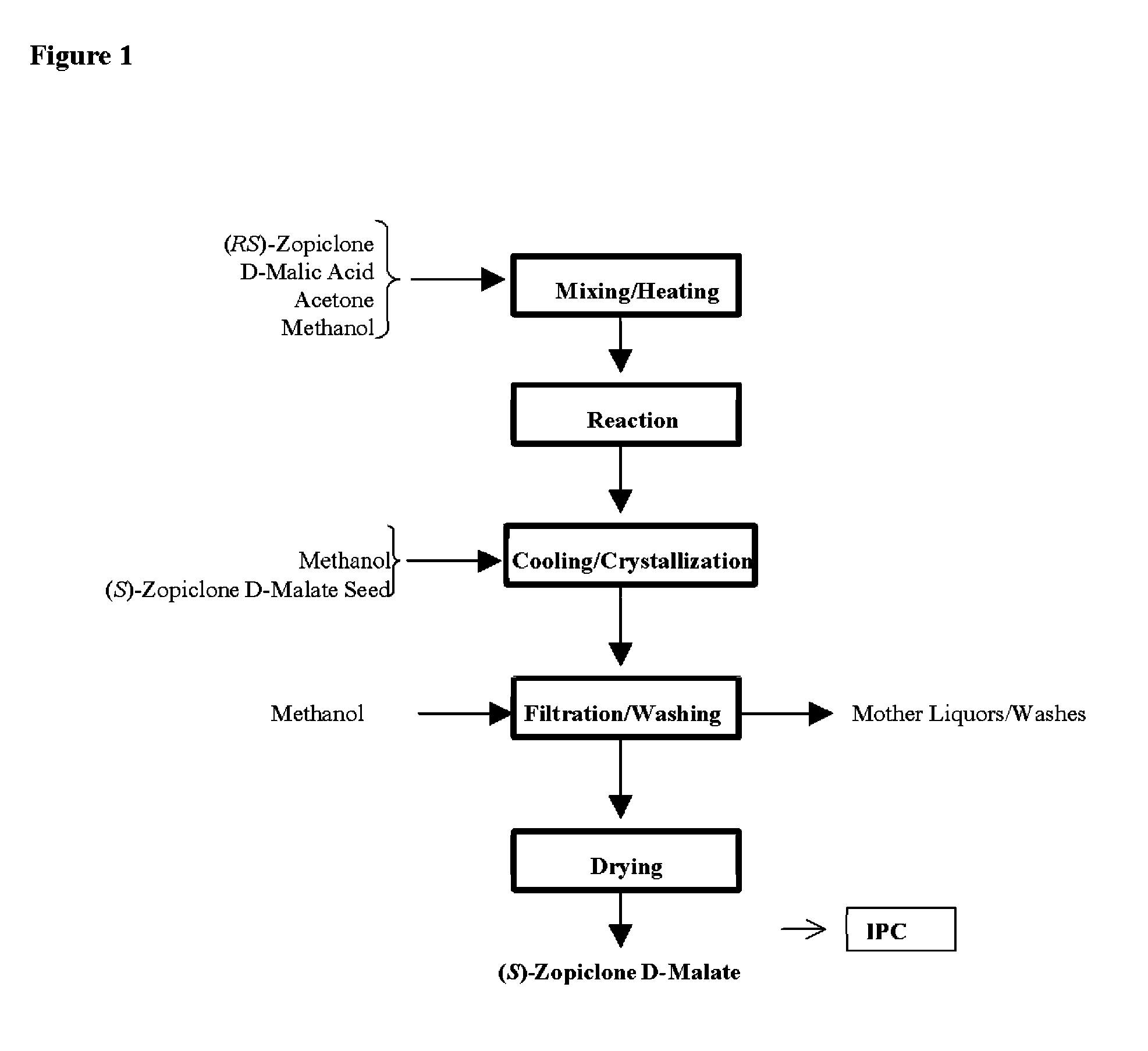
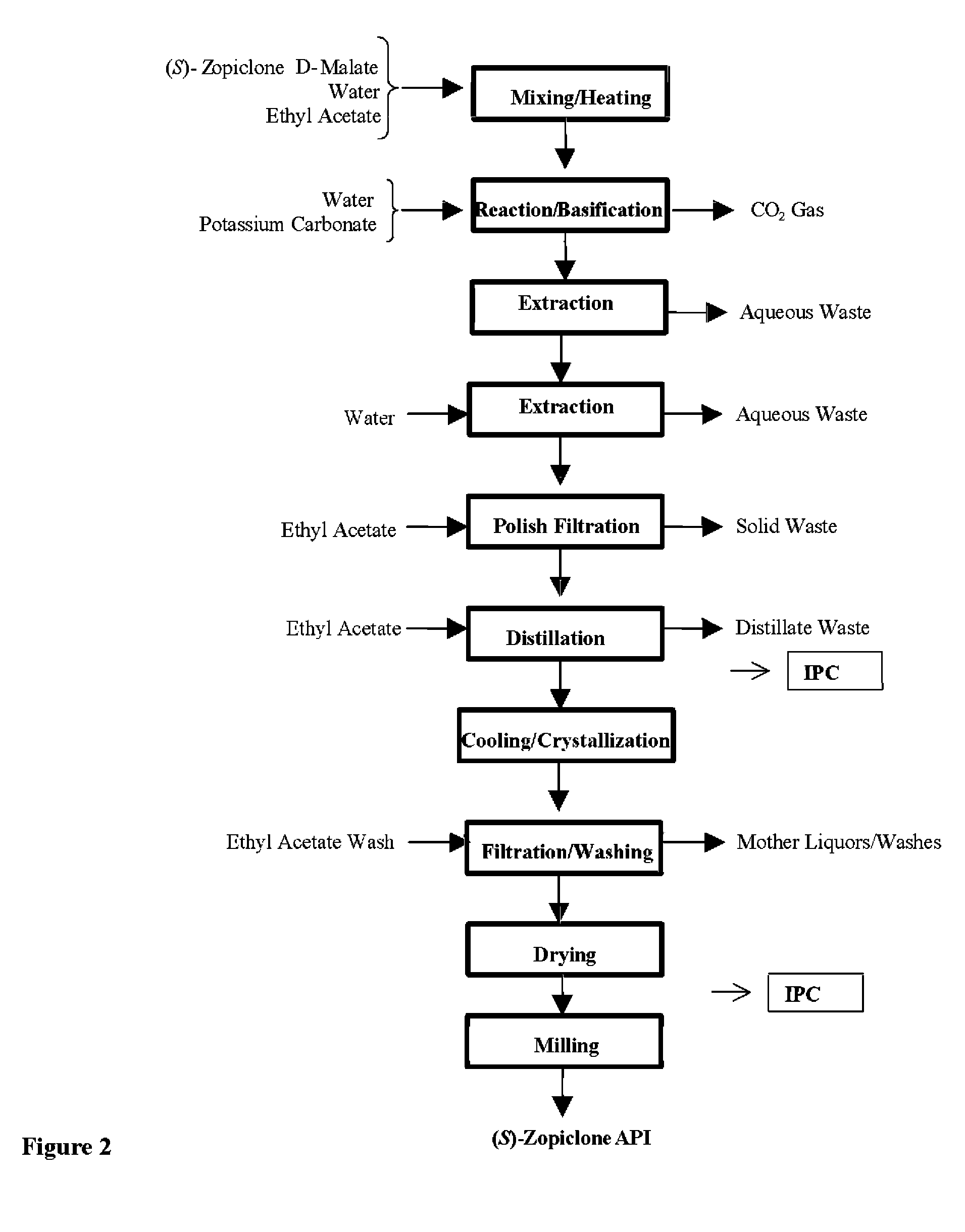
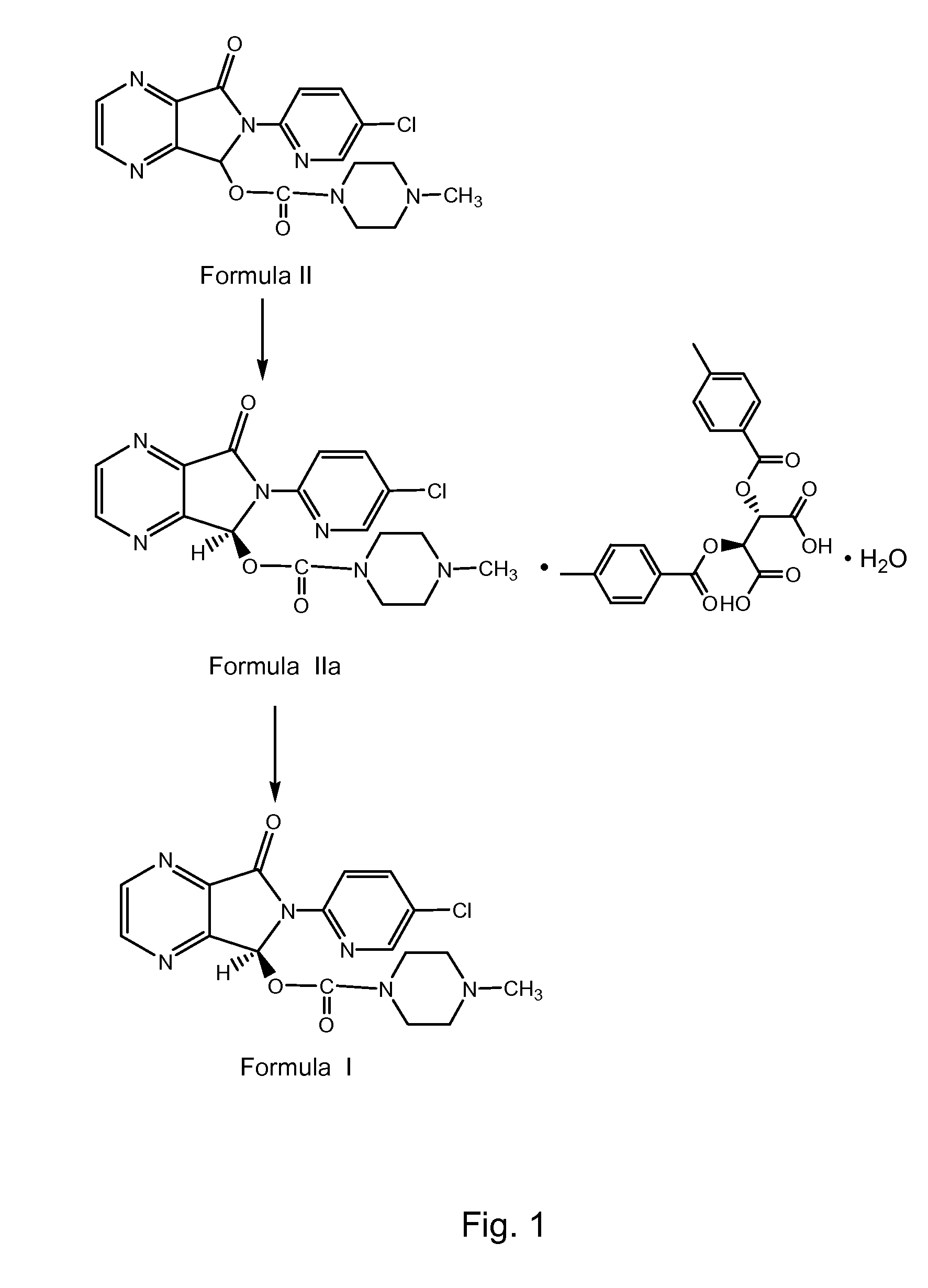
Resolution of zopiclone is carried out by reacting zopiclone with D-(+)-malic acid to obtain D-(+)malic acid S-zopiclone, dissolving it into water, adjusting pH, precipitating out crystal, extracting by acetic ether, drying acetic ether layer, distilling to remove acetic ether and obtaining white crystal.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
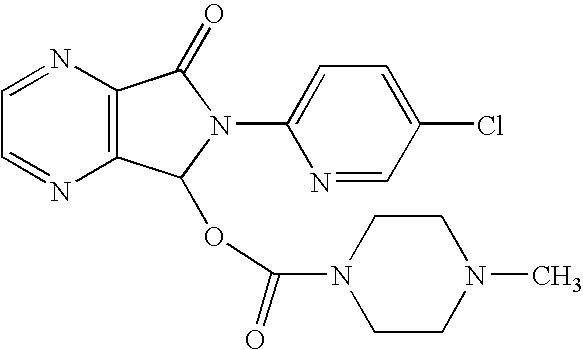
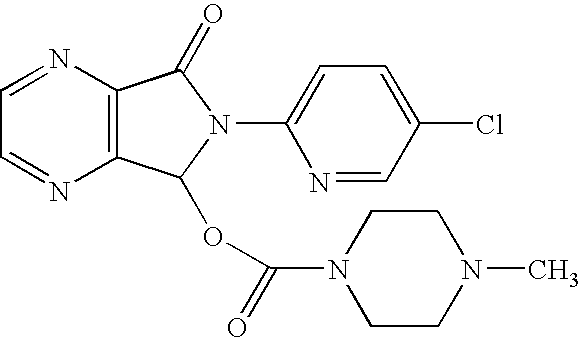
Process for the preparation of eszopiclone
InactiveUS20080146800A1Minimizes problemEfficient and cost-effective processOrganic chemistryMetaclazepamPyrazine
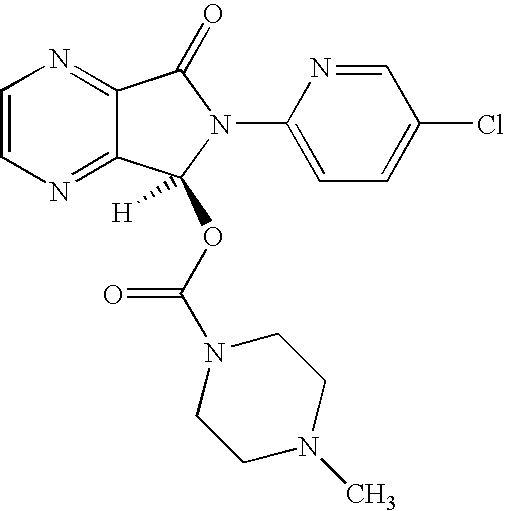
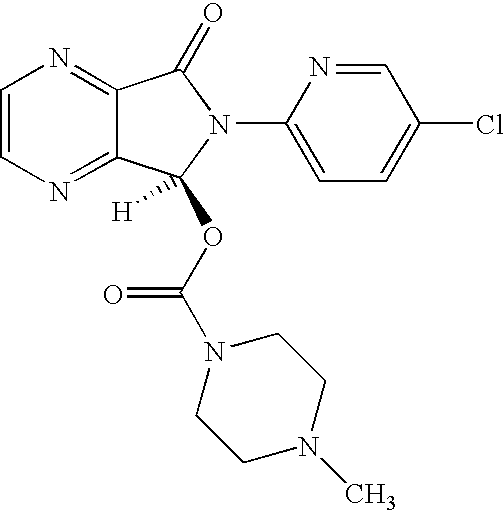
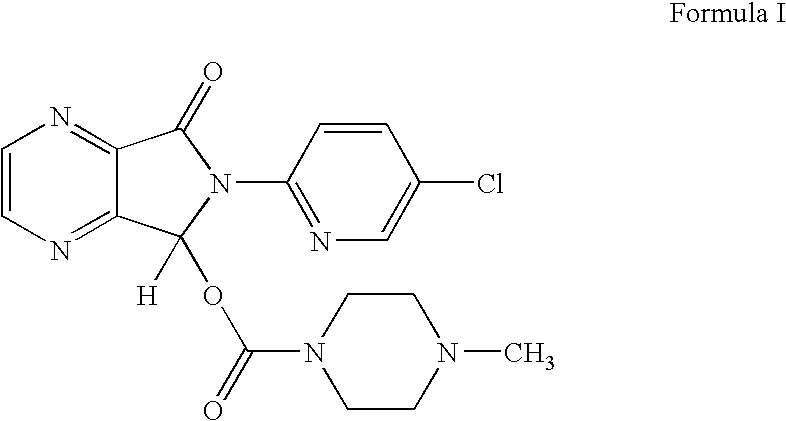
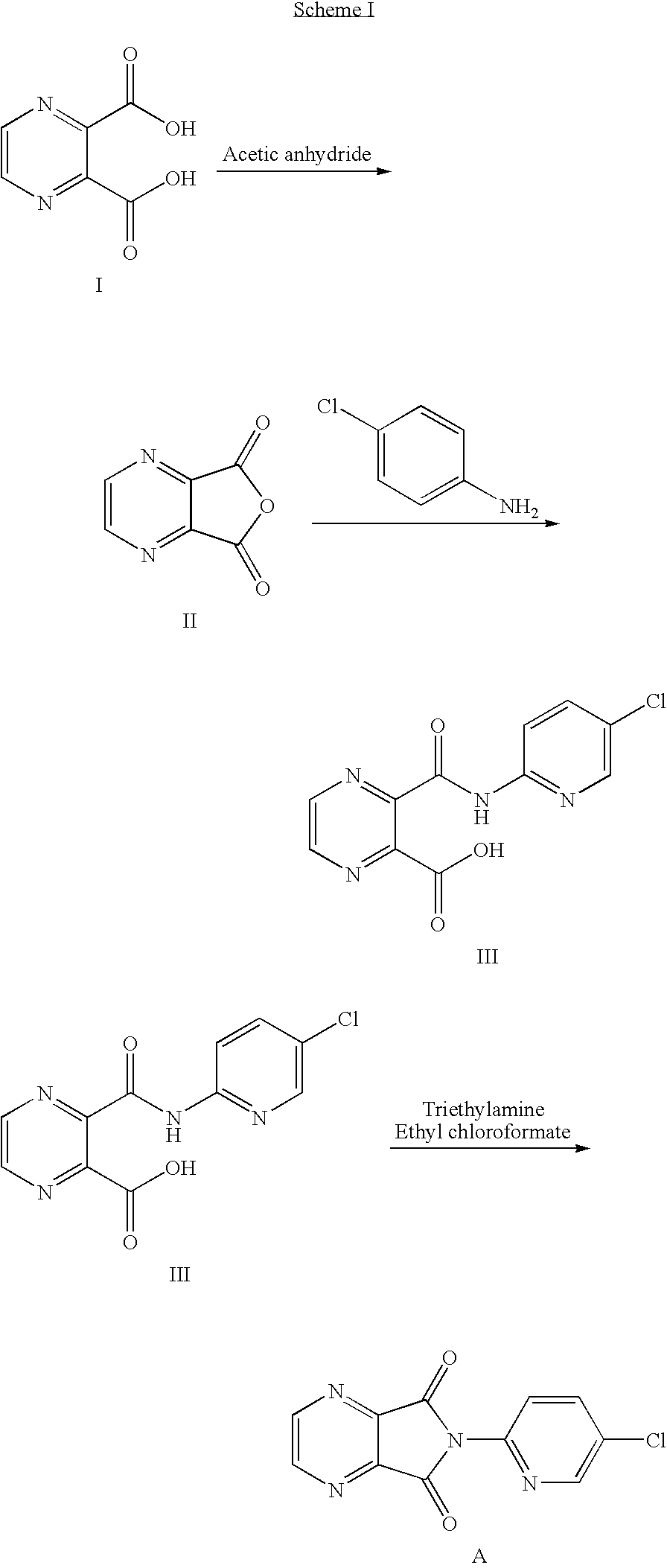
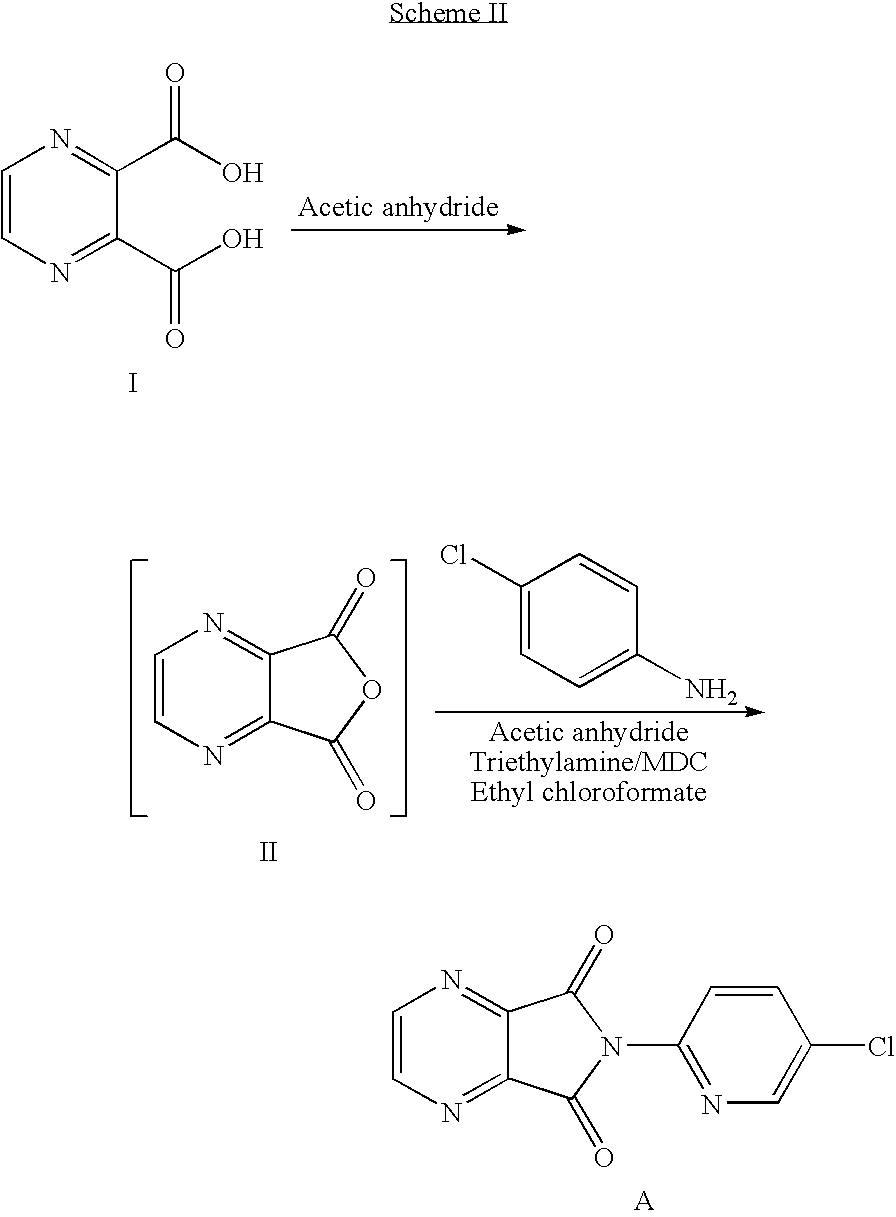
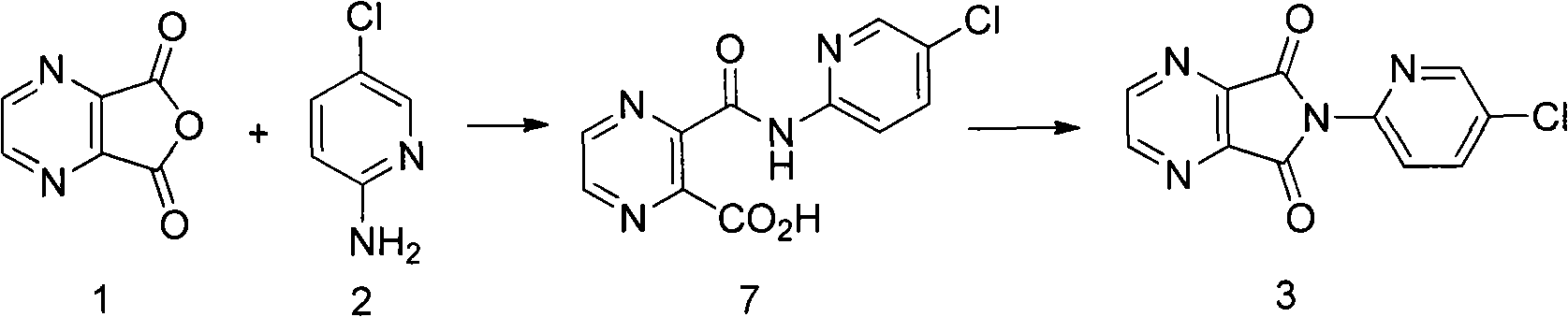
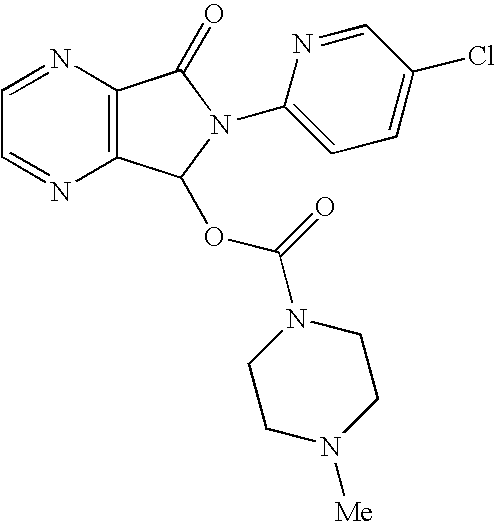
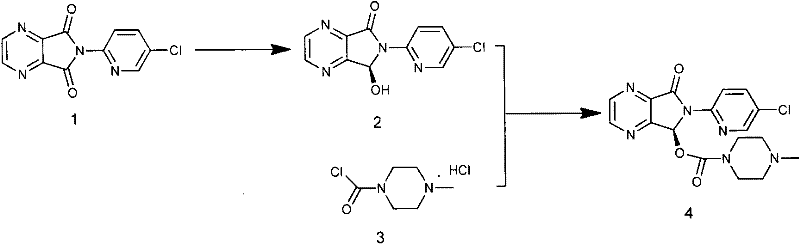
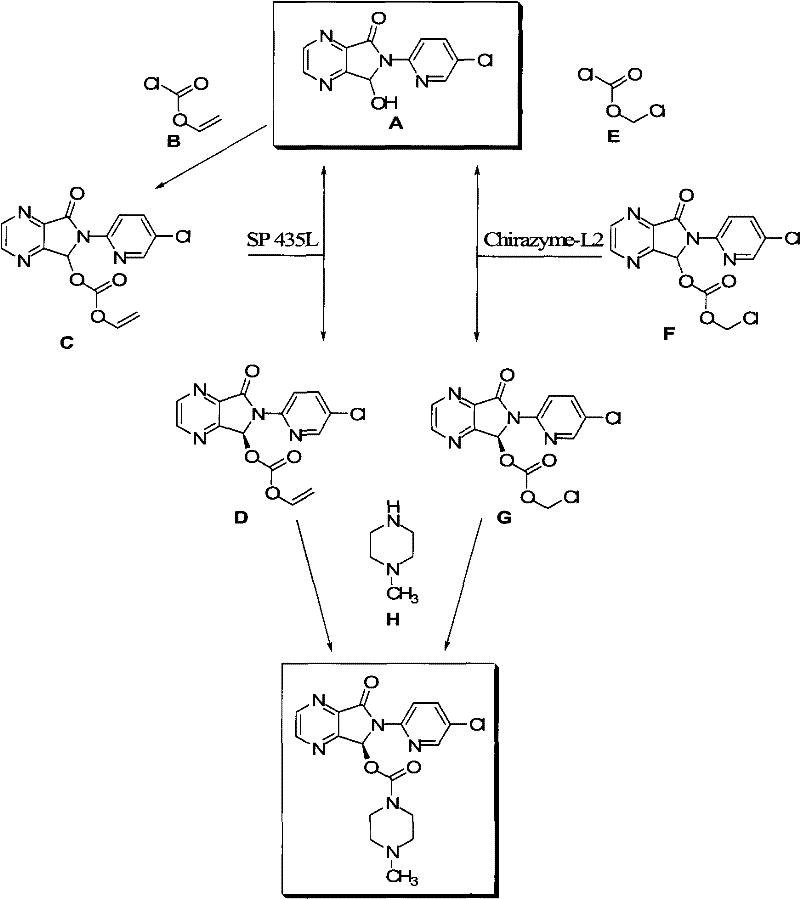
The invention relates to a process for making of 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo-[3,4-b] pyrazin-5-yl-4-methyl piperazine-1-carboxylate, also known as zopiclone. The invention further describes an effective method for resolving of zopiclone into its enantiomers (eszopiclone and (R)-zopiclone) and also provides a method of recycling of (R)-zopiclone.
Owner:CENTAUR CHEM PVT +1
Compositions comprising zopiclone derivatives and methods of making and using the same
ActiveUS20040147521A1Convenient treatmentReduces and avoids symptomBiocideOrganic chemistryZopicloneMedicinal chemistry
Owner:WOODWARD SPECIALTY LLC
Eszopiclone preparation method
The invention relates to an eszopiclone preparation method comprising main steps that: zopiclone is subjected to a reaction with D-dibenzoyltartaric acid or a hydrate thereof, such that dextral zopiclone-D-dibenzoyltartrate is produced; and the salt is dissociated, such that eszopiclone is obtained. According to the method, D-dibenzoyltartaric acid with a substance amount of a quarter to a half of that of zopiclone is adopted. The reaction conditions are mild, operation is convenient, product yield is high, and product purity is high. The method is suitable for large-scale industrialized productions.
Owner:四川弘远药业有限公司
Preparation method of zopiclone
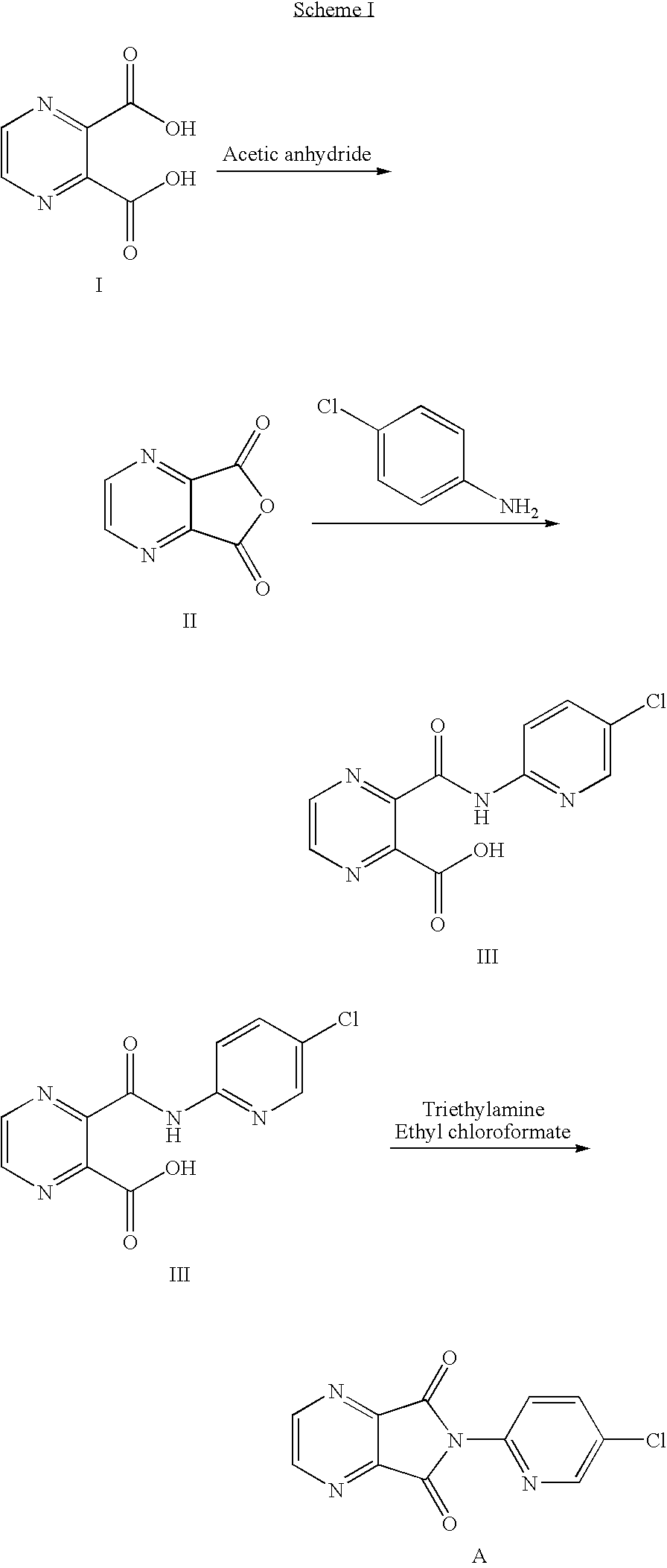
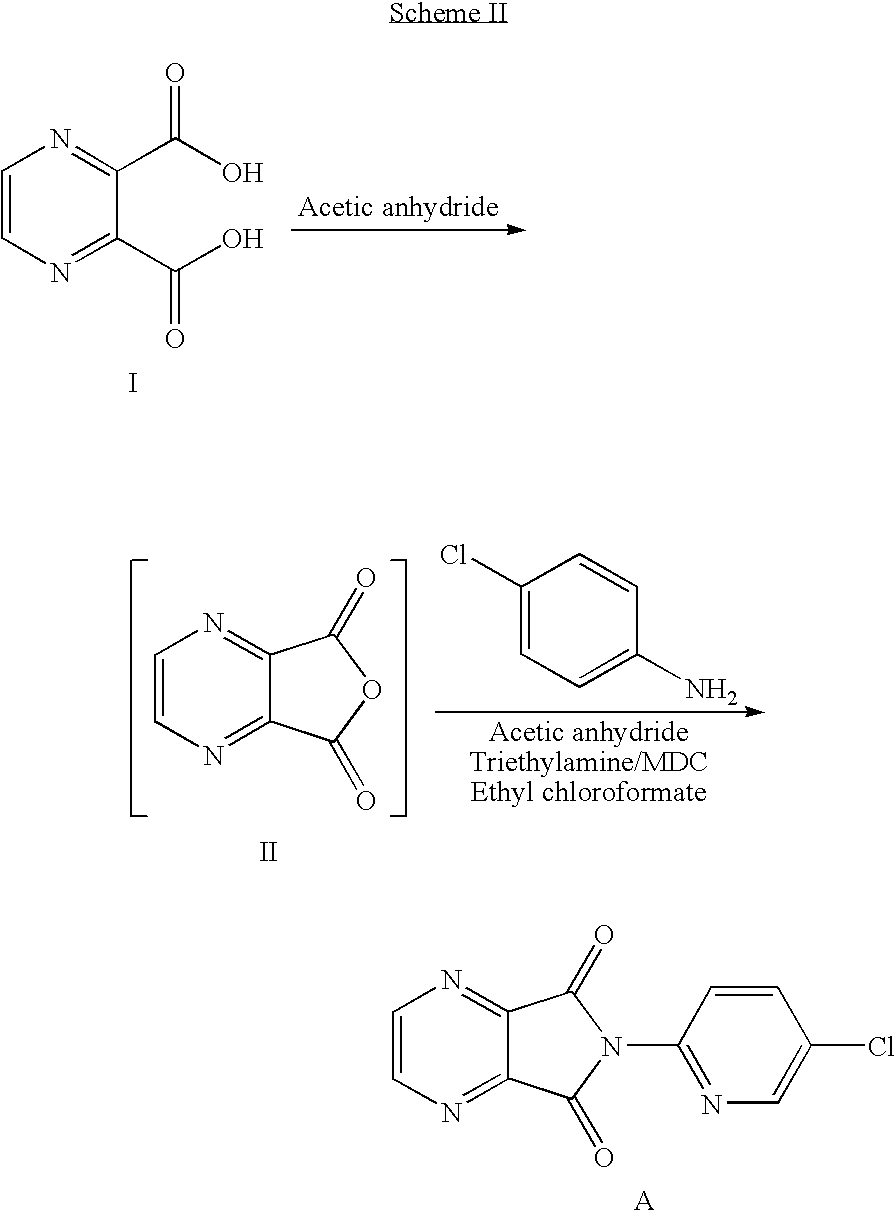
ActiveCN103664952AReduce usageEmission reductionOrganic chemistryEthyl chloroformateAcetic anhydride
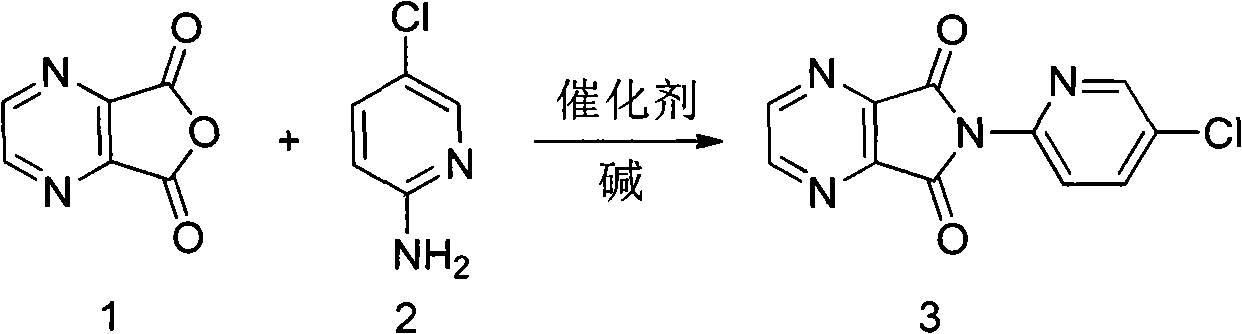
The invention relates to a preparation method of zopiclone for improving sleeping, belonging to the field of medicines. In the preparation process of 6-(5-chlro-2-pyridyl)-5, 7-dioxo-5, 6-dihydropyrrolo[3, 4-b] pyrazine, namely a compound 3, by taking DMAP (dimethylaminopyridine) as a catalyst, in the presence of triethylamine, cyclization is directly carried out to synthesize an intermediate 3. The crude product yield is 85%, the yield is improved, the operation is simplified, and irritant reagents such as acetic anhydride, thionyl chloride, ethyl chloroformate and the like are not used, thereby facilitating production, facilitating recovery of xylene as a solvent and reducing emission of three wastes. Zopiclone is further synthesized by the compound 3. The method is concise in whole line, simple and convenient to operate and more suitable for industrialized production.
Owner:迪嘉药业集团股份有限公司
Methods for preparing eszopiclone crystalline form a, substantially pure eszopiclone and optically enriched eszopiclone
The present invention provides methods for preparing eszopiclone Form A, substantially chemically pure eszopiclone, or eszopiclone with low level(s) of residual solvent(s). The present invention also provides eszopiclone with low level(s) of residual solvent(s). The present invention also provides a process for optical enrichment of eszopiclone free base. For instance, one of the embodiments of the invention is directed to a method of preparing eszopiclone Form A, wherein the method comprises crystallizing eszopiclone free base from a solvent selected from the group consisting of isopropanol (IPA), methyl isobutyl ketone (MIBK), acetone, n-butanol, i-butanolisobutanol, 2-butanol, tetrahydrofuran (THF), dimethyl carbonate, methanol, ethanol, ethyl lactate, dimethylformamide (DMF), carbon tetrachloride, toluene, iso-butyl acetate and mixtures thereof.
Owner:TEVA PHARM USA INC
Treatment of obstructive sleep apnea syndrome with a combination of a carbonic anhydrase inhibitor and an additional active agent
InactiveUS20110224196A1Amelioration of the extent of each apneaAlleviate excessive daytime sleepinessBiocideNervous disorderZaleplonActive agent
This invention relates generally to methods and pharmaceutical formulations useful in treating patients suffering from obstructive sleep apnea syndrome (OSAS). Treatment of OSAS is effected by administering a carbonic anhydrase inhibitor to the patient in combination with at least one additional active agent. Examples of additional active agents include modafinil, eszopiclone, zolpidem, zaleplon, and phentermine.
Owner:VIVUS
Eszopiclone process
InactiveUS7476737B2Simple, efficient, inexpensive, ecofriendlyImprove scalabilityBiocideOrganic chemistryPyrazinePyridine
Owner:DR REDDYS LAB LTD +1
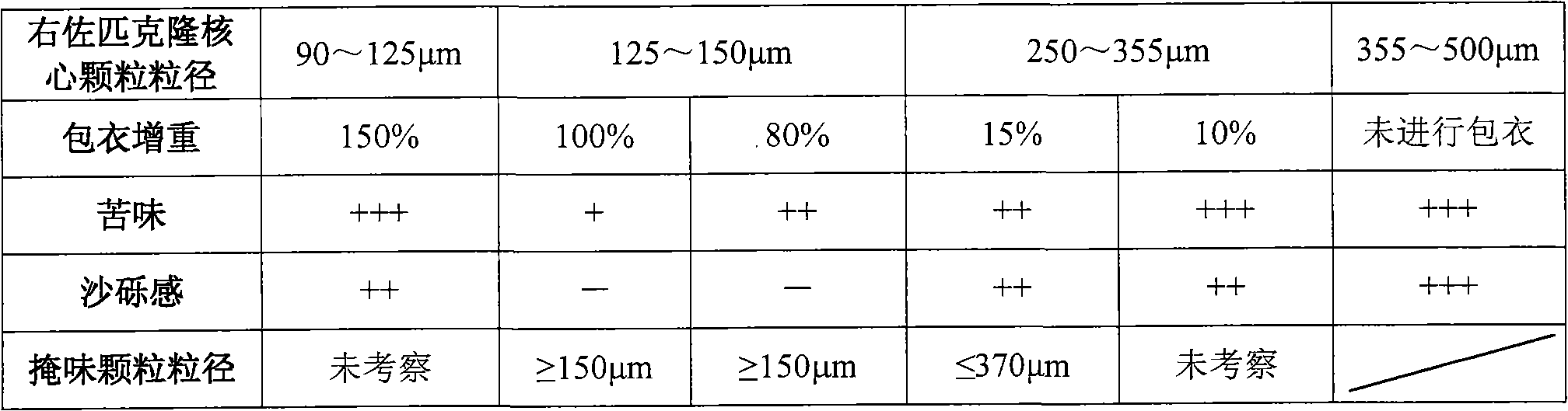
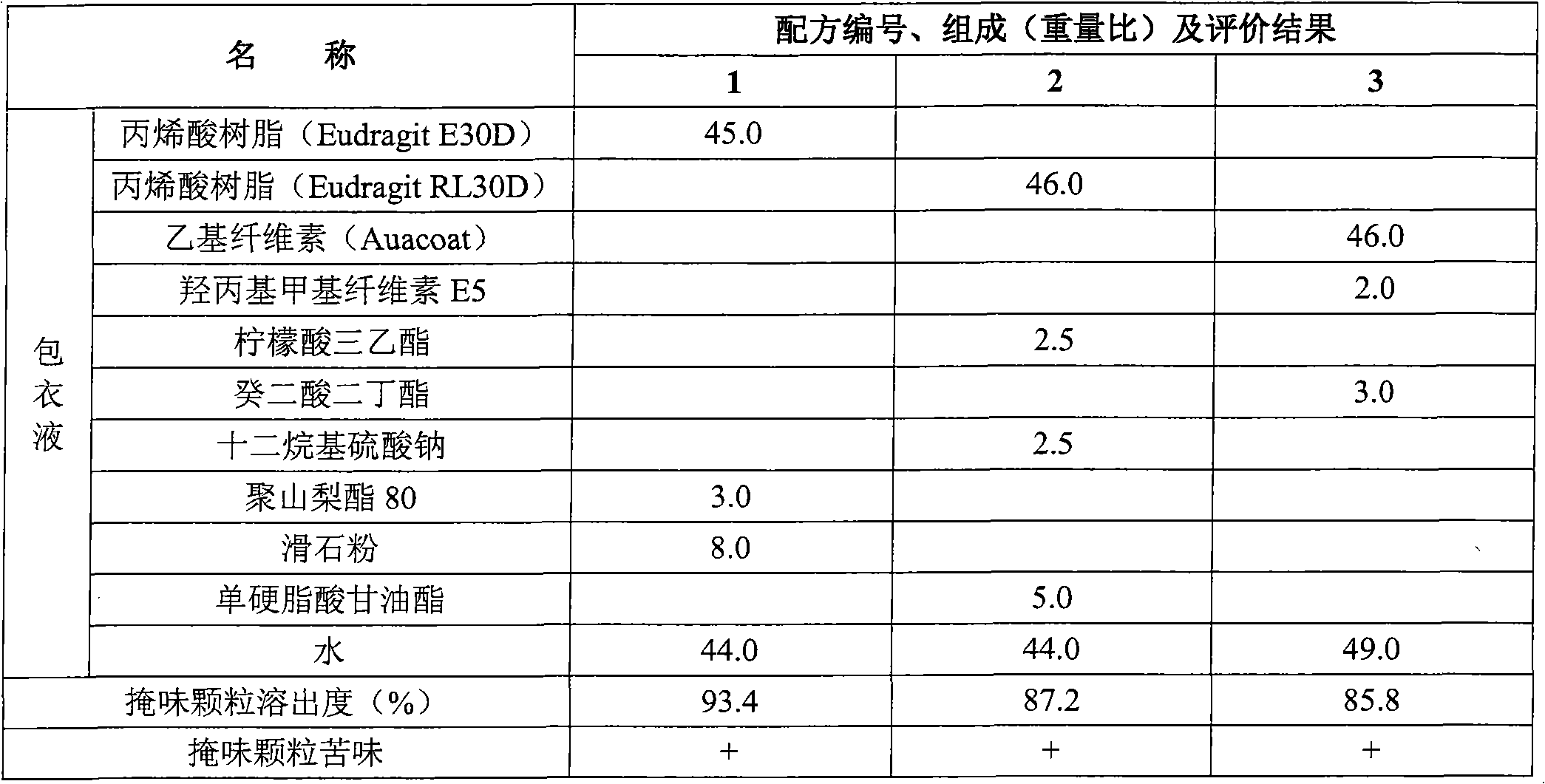
Eszopiclone-containing particle and its preparation method
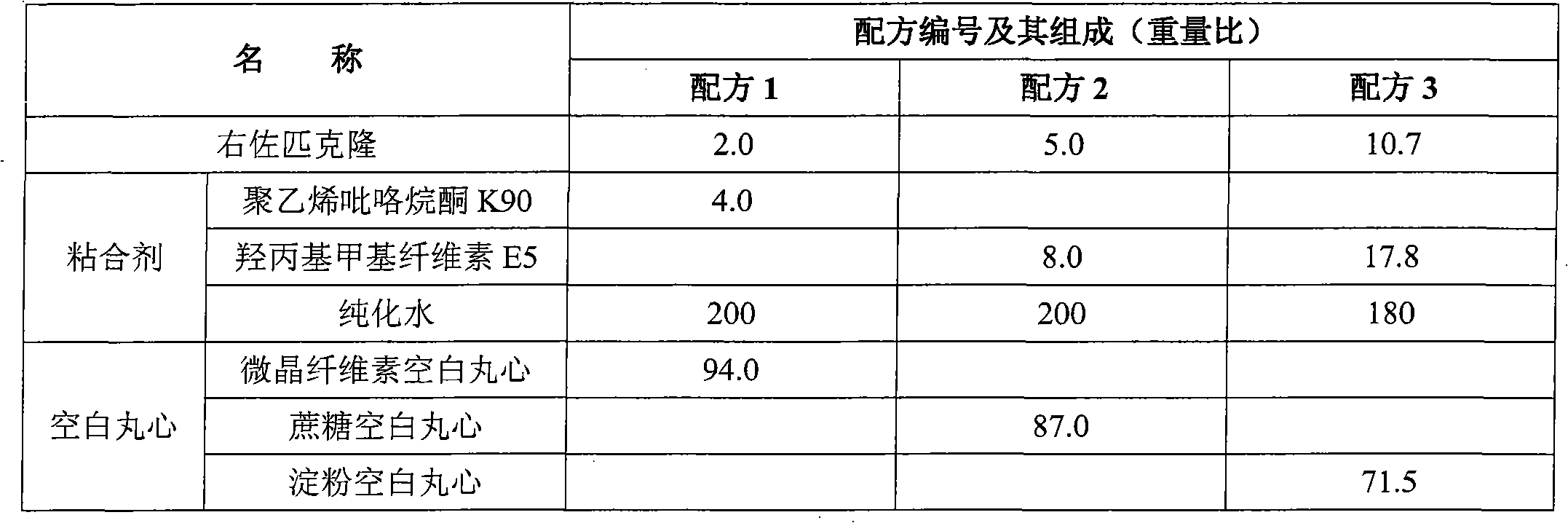
ActiveCN102727452AAdvantages of preparation processImprove use complianceOrganic active ingredientsNervous disorderOrally disintegrating tabletDissolution
The invention relates to an eszopiclone-containing particle and its preparation method. The particle can cover the bitter taste of eszopiclone, has no obvious sandy feeling, has uniform contents and high dissolution rate, and can be used for preparing solid dosage forms such as orally disintegrating tablets, dispersing tablets, suspension granules and the like.
Owner:CHENGDU KANGHONG PHARMA GRP
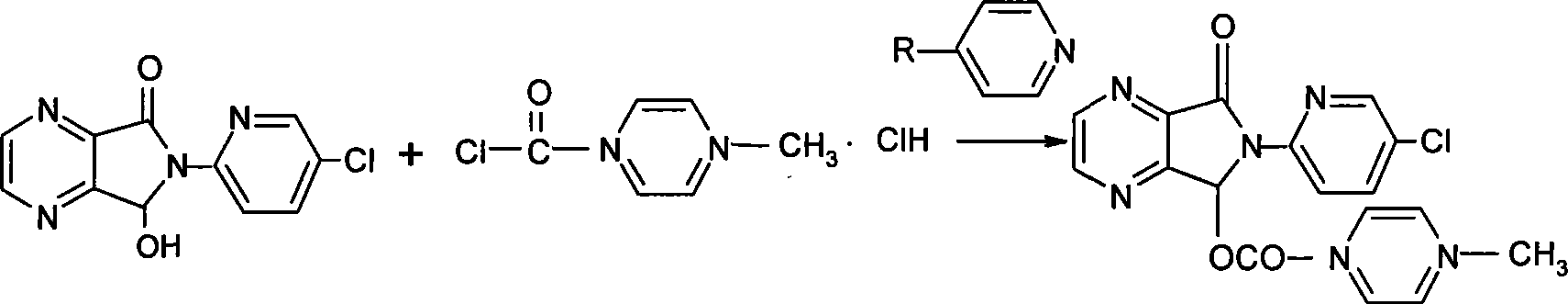
Method for producing zopiclone
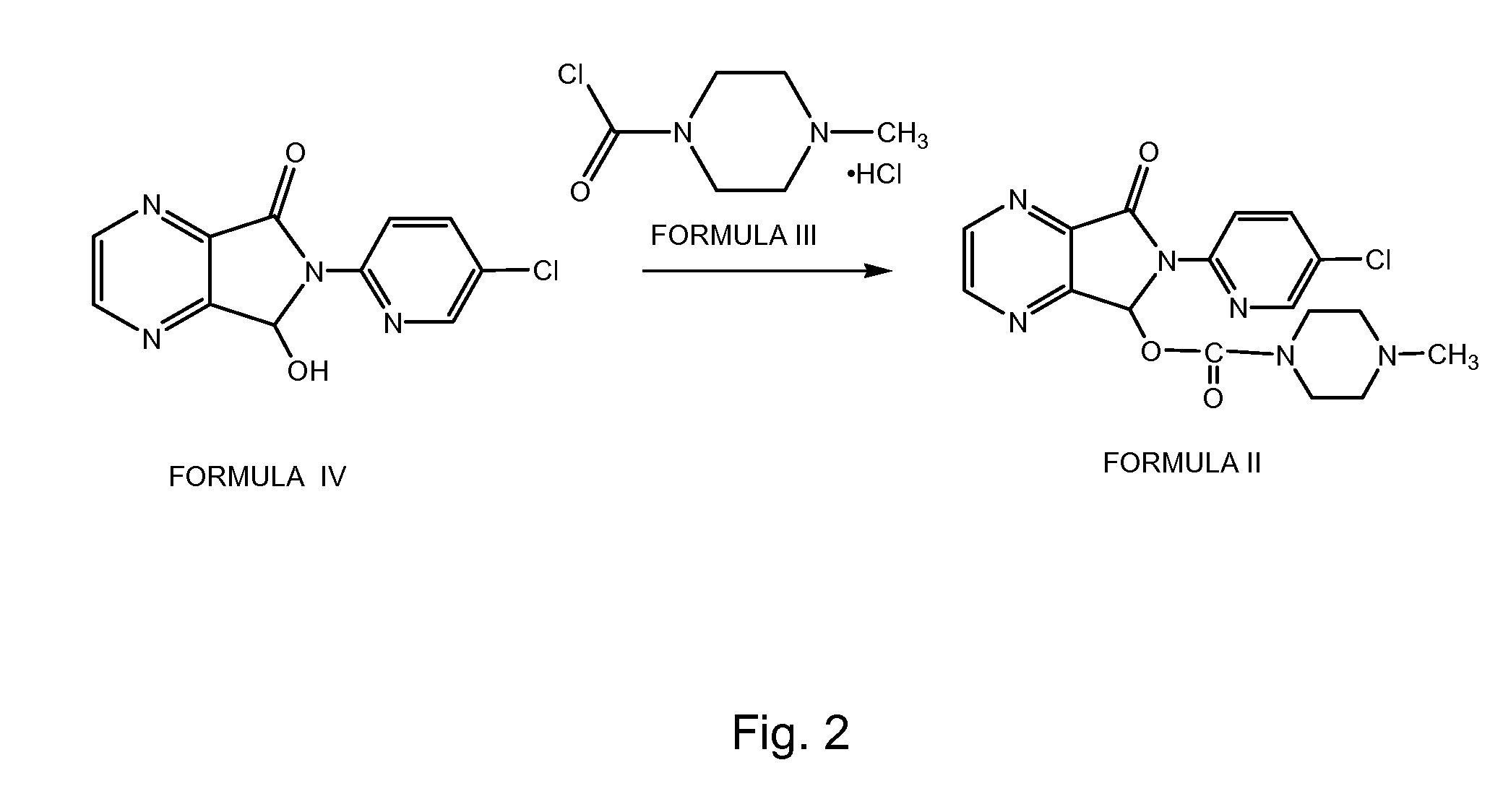
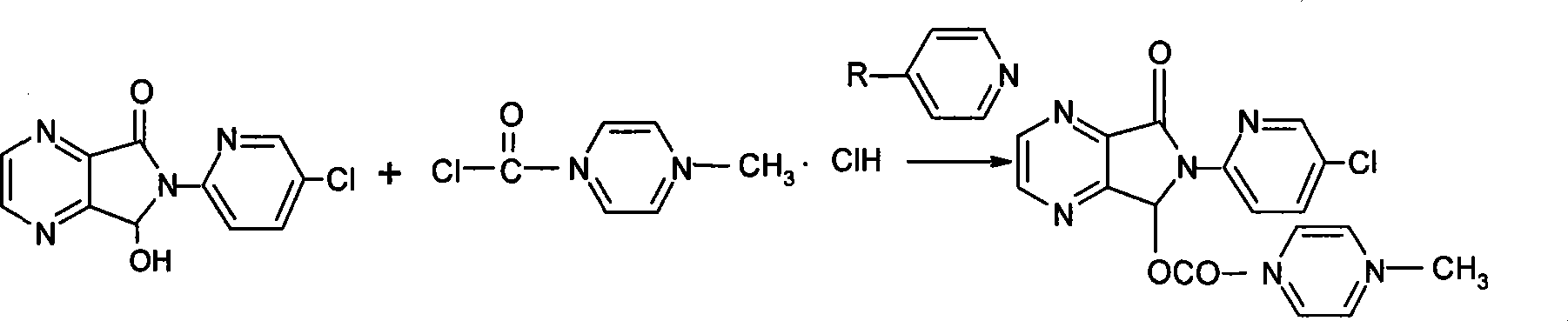
A preparation method of zopiclone uses 6-(5-chlorin-2-pyridyl)-5-hydroxy-7-oxo-5, 6-dihydropyrrole [3, 4-b] pyrazine and 1-chlorocarbonyl-4-methylpiperazine hydrochloride as material, in anhydrous organic solvent and alkali condition, uses 4-substituent pyridine as catalyst to prepare the product zopiclone. The inventive method uses the organic solvent with a little toxin and unease burning explosion, via special catalyst to process reaction completely, while the product has high purity and yield.
Owner:QILU PHARMA HAINAN +1
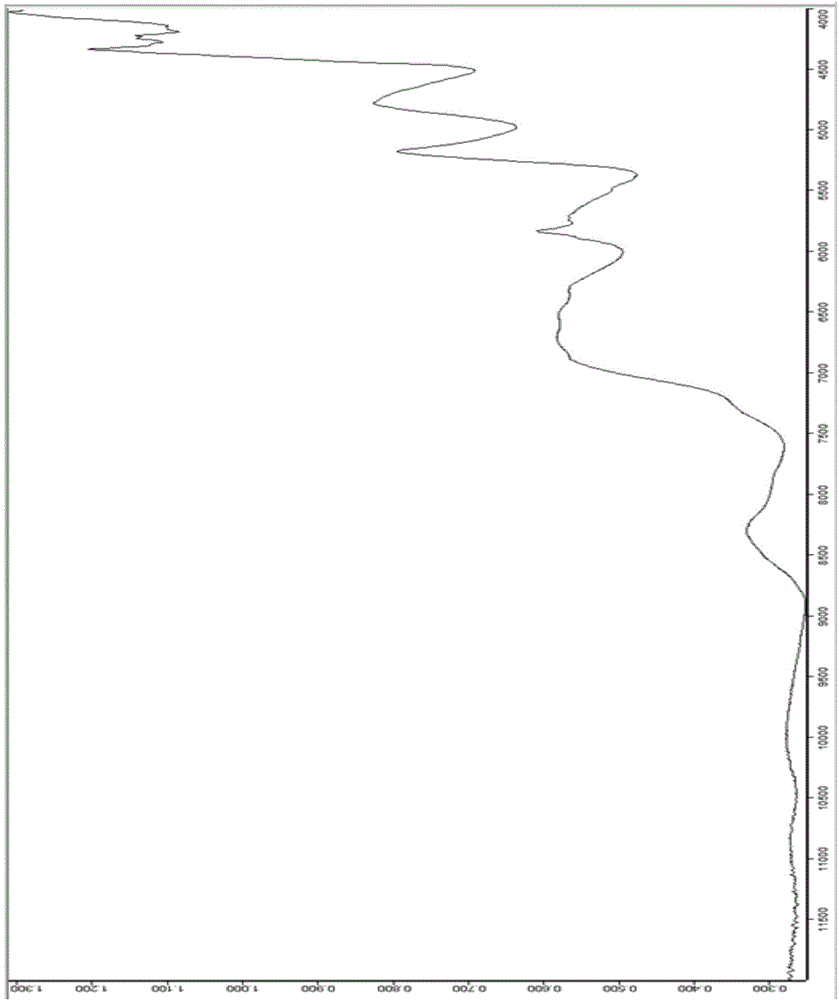
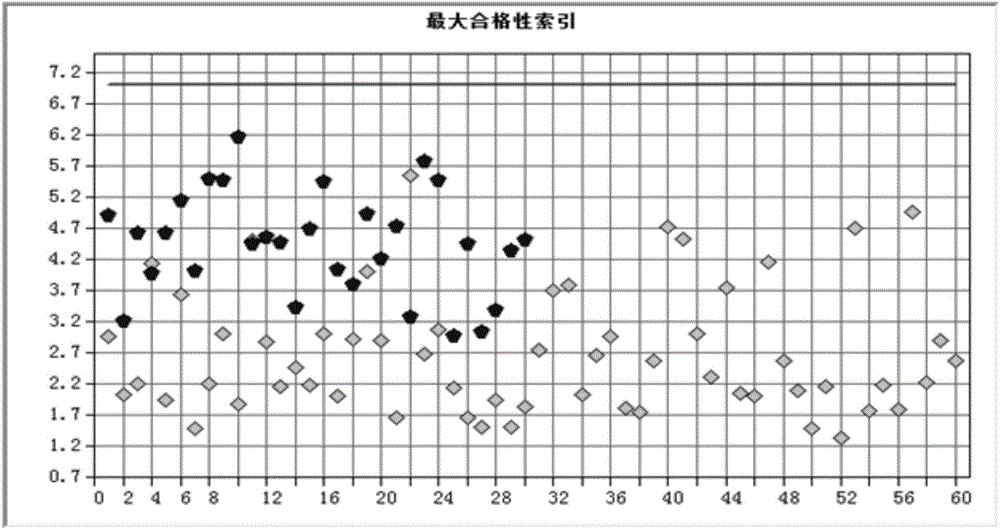
Zopiclone tablet rapid identification method based on near infrared spectrum
InactiveCN104596983AResolve accuracySolve quicklyMaterial analysis by optical meansReflectance spectroscopyPass rate
The invention provides a zopiclone tablet rapid identification method based on a near infrared spectrum. The zopiclone tablet rapid identification method comprises the following steps: firstly, acquiring an original graph and pre-processing the original graph; modeling by adopting two instruments, and identifying by one instrument, thus realizing modeling and identifying a spectrum by using the instruments; and carrying out a qualification test on near infrared diffuse reflection spectrums of a plurality of batches of dynamic production samples by using a model so as to judge whether medicines are counterfeited or not. After the model is established, a negative sample and a positive sample are used for testing the stability of the model respectively; and when the result is that the passing rate of the positive sample is 100% and the negative sample does not pass, the false positive rate and the false negative rate of the model are relatively low, the method is suitable for market rapid checking and the result is reliable. A rapid quality identification and evaluation method for zopiclone tablets which are produced by Qilu Pharmaceutical Co.,Ltd. and are packaged by an aluminum-plastic plate is established, the detected sample does not need to be subjected to complicated pre-treatment, and the sample is not damaged and polluted; and a lot of analysis time and cost can be saved, and the method is a convenient, rapid and lossless green analysis technology.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Compositions comprising zopiclone derivatives and methods of making and using the same
InactiveUS20070049590A1Minimizing spread and worseningGood curative effectBiocideOrganic chemistryMedicinal chemistryZopiclone
Owner:WOODWARD SPECIALTY LLC
Sedative-hypnotic preparation, compound preparation, preparation method and pharmaceutical composition thereof
ActiveCN103919780AImprove stabilityQuality assuranceOrganic active ingredientsNervous disorderOrganic acidSedative/hypnotic
The invention relates to a sedative-hypnotic preparation, a compound preparation, a preparation method and a pharmaceutical composition thereof. The compound preparation of the sedative-hypnotic preparation includes zopiclone or eszopiclone and a pharmaceutically acceptable auxiliary material which includes a stabilizing agent with a dosage being, by mass, 0.1%-10% of the zopiclone or the eszopiclone. The stabilizing agent is one or more of a pharmaceutically acceptable organic acid salt, a pharmaceutically acceptable organic acid buffer pair and a pharmaceutical acceptable anti-oxidant. The pharmaceutical composition of the sedative-hypnotic preparation forms a compound preparation pharmaceutical composition with other pharmaceutical active substances. The preparation method of the sedative-hypnotic preparation or the compound preparation includes carrying out a wet granulation process to the pharmaceutical composition of the sedative-hypnotic preparation or the compound preparation pharmaceutical composition. The sedative-hypnotic preparation or the compound preparation is good in stability and is excellent in dissolution rate and content uniformity. Quality of the preparations can be ensured and the preparations are suitable for industrial production.
Owner:SHANGHAI ZHONGXI PHARMA
Novel Process
The present invention relates to a process for optically resolving eszopiclone, comprising chiral chromatography. Preferably the process comprises a multi-column continuous process or a simulated moving bed process. Preferably the stationary phase used in the chiral chromatography process comprises an amylose or cellulose derivative of tris(3,5-dimethylphenyl carbamate), or an amylose derivative of tris-α-methylbenzylcarbamate. The process of the present invention has the advantage that it is high yielding and can be carried out on an industrial scale.The present invention also provides eszopiclone, or a pharmaceutically acceptable salt thereof, obtained by the chiral chromatography process. The eszopiclone or salt thereof is suitable for use as a medicament, for example, for the treatment of anxiety or insomnia.
Owner:GENERICS UK LTD
Chiral synthesis of Eszopiclone
InactiveCN102675318AAtom utilization is highReasonable designOrganic chemistrySynthesis methodsPyrazine
The invention relates to a chiral synthesis method for Eszopiclone and belongs to the technical field of medicine. The chiral synthesis method for Eszopiclone comprises the following two steps of reaction: (1), reducing under the action of a 6-(5-chlorine-2-pyridyl)-5,6-dioxo-6,7-dihydro-5H-pyrrol(3,4-b)pyrazine chiral reagent to obtain 6-(5-chlorine-2-pyridyl)-5(S)-hydroxyl-7-oxo-6,7-dihydro-5H-pyrrol(3,4-b)pyrazine; and (2), reacting the 6-(5-chlorine-2-pyridyl)-5(S)-hydroxyl-7-oxo-6,7- dihydro-5H-pyrrol(3,4-b)pyrazine with 1-chloroformyl-4-methylpiperazine hydrochloride under the action of organic base to obtain the Eszopiclone. The chiral synthesis method for the Eszopiclone has the advantages of high atom utilization ratio, short steps and advanced technology.
Owner:SHANGHAI ZNBIOCHEM
Eszopiclone solid preparation and preparation method thereof
ActiveCN102106824AEasy to operateImprove securityOrganic active ingredientsNervous disorderMechanical crushingZopiclone
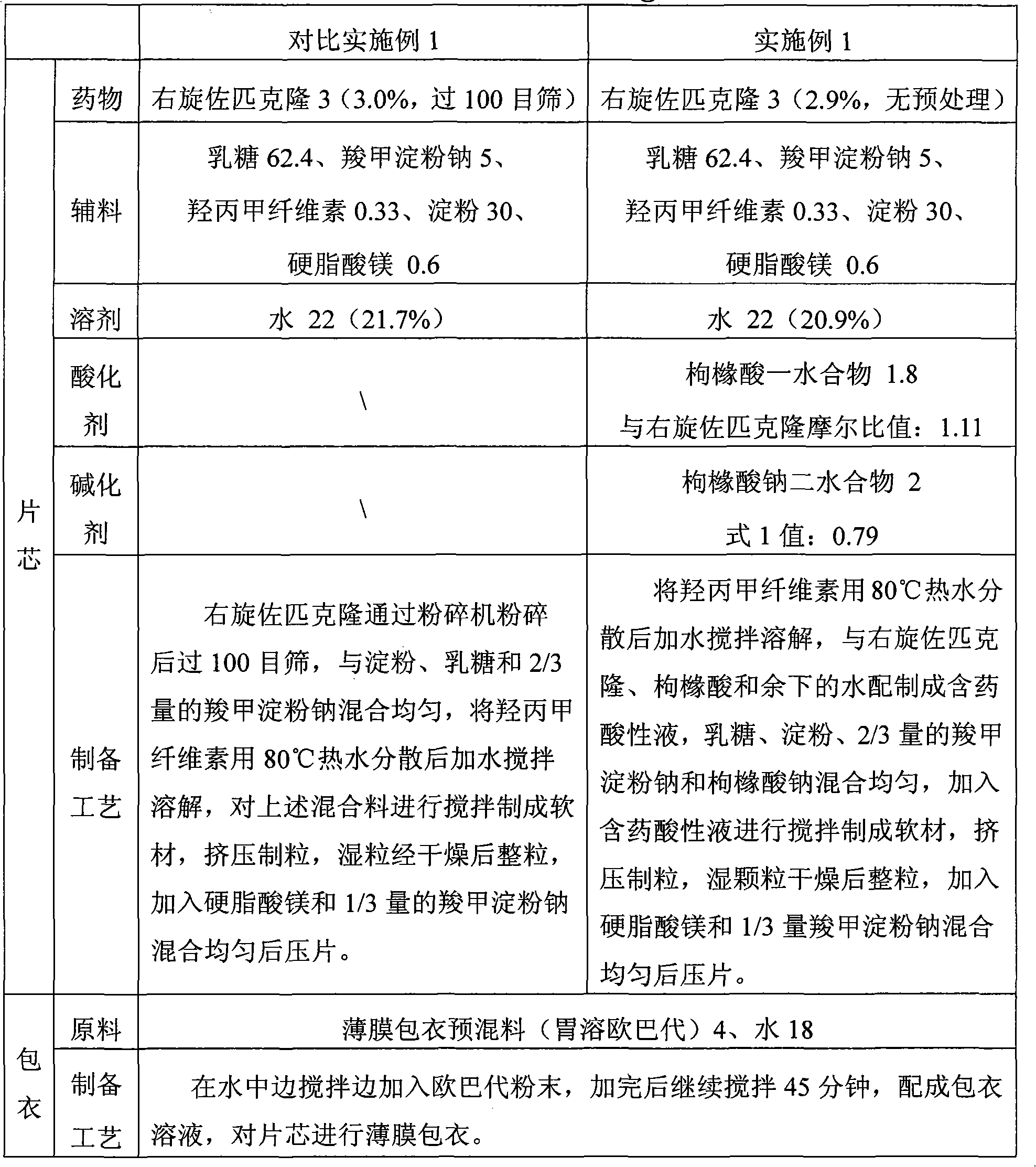
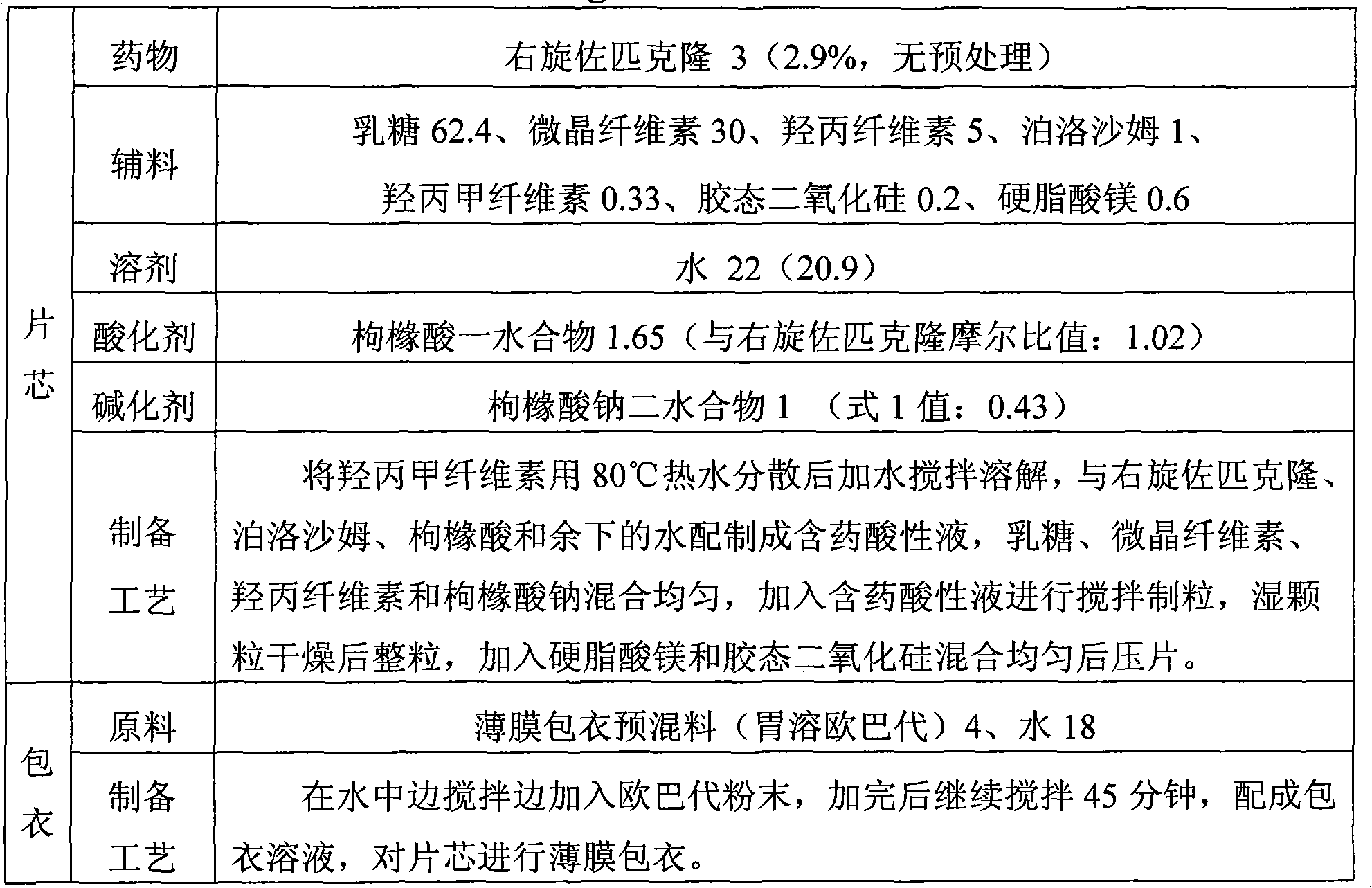
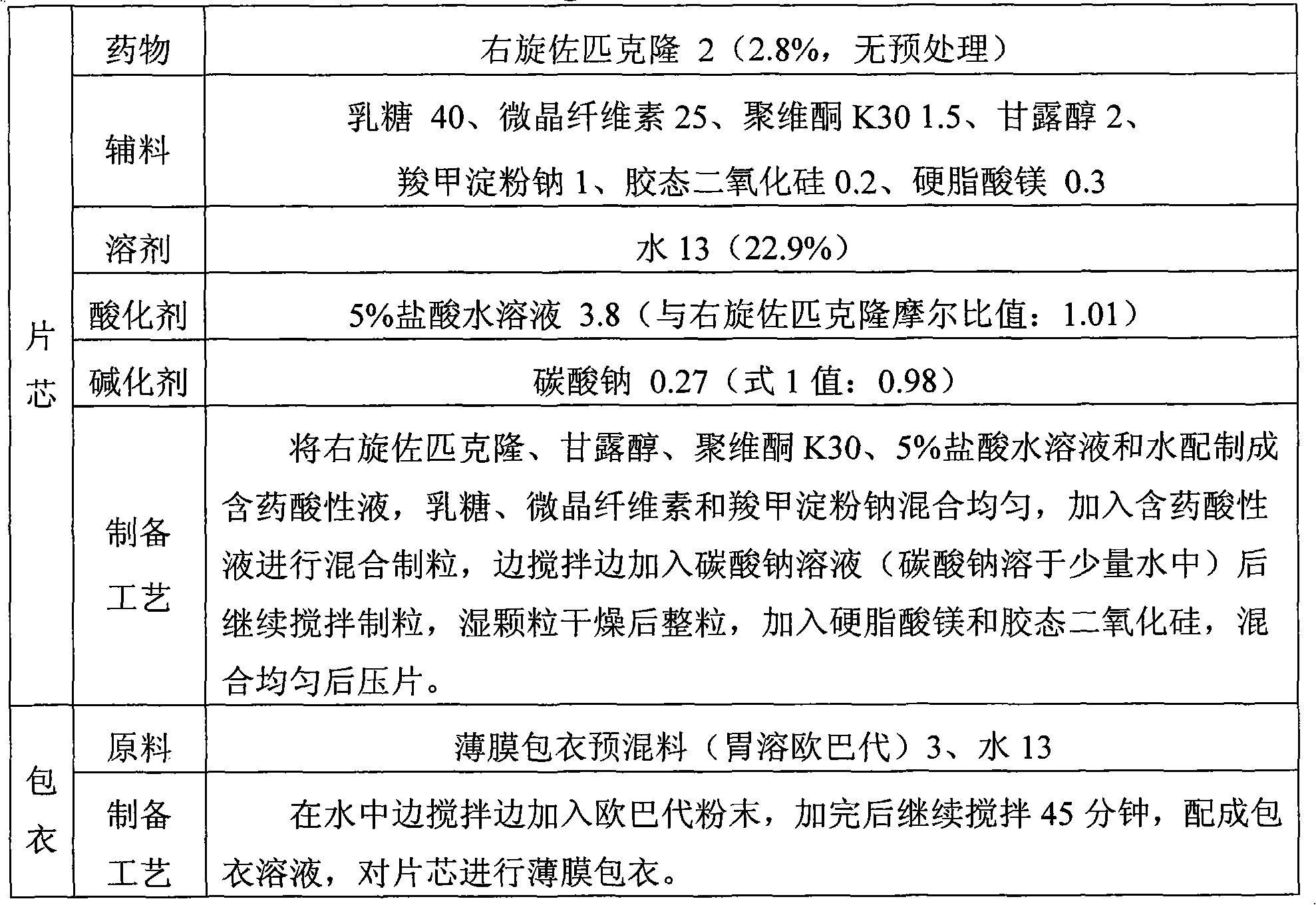
The invention discloses the preparation method of eszopiclone solid preparation, which comprises the following steps of dissolving eszopiclone into acidic solution containing an acidifying agent to obtain medicine-containing acidic solution; and uniformly mixing alkalinizing agent, accessories and the medicine-containing acidic solution to granulate by a wet method, wherein the alkalinizing agentis agent by which the acidity of mixed solution of the alkalinizing agent and the medicine-containing acidic solution is reduced relative to the acidity of the medicine-containing acidic solution. The invention also discloses eszopiclone solid preparation prepared by the method. According to the method disclosed by the invention, the defects of the serious pollution, high loss and serous potential safety hazards brought by mechanical crushing treatment are avoided; and the method is simple, convenient and feasible for operation and easy for industrial production and has high safety coefficient. The eszopiclone solid preparation prepared by the method has the advantages of excellent dissolution property, stability, and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Process for the preparation of eszopiclone
InactiveUS7786304B2Minimizes problemEfficient and cost-effective processOrganic chemistryPyrazineAcyl group
Owner:CENTAUR CHEM PVT +1
Method for resoluting zopiclone
Resolution of zopiclone is carried out by reacting zopiclone with D-(+)-malic acid to obtain D-(+)malic acid S-zopiclone, dissolving it into water, adjusting pH, precipitating out crystal, extractingby acetic ether, drying acetic ether layer, distilling to remove acetic ether and obtaining white crystal.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Racemization method of eszopiclone
The invention relates to a racemization method of eszopiclone. According to the invention, tetramethylguanidine which is cheap and easily available is used as a racemization agent. The method provided by the invention is simple to operate, is safe and stable, has advantages of high yield and good product quality, and is suitable for industrial production.
Owner:四川弘远药业有限公司
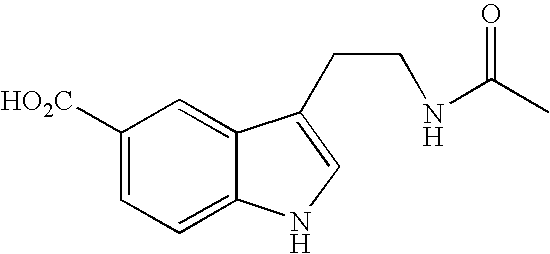
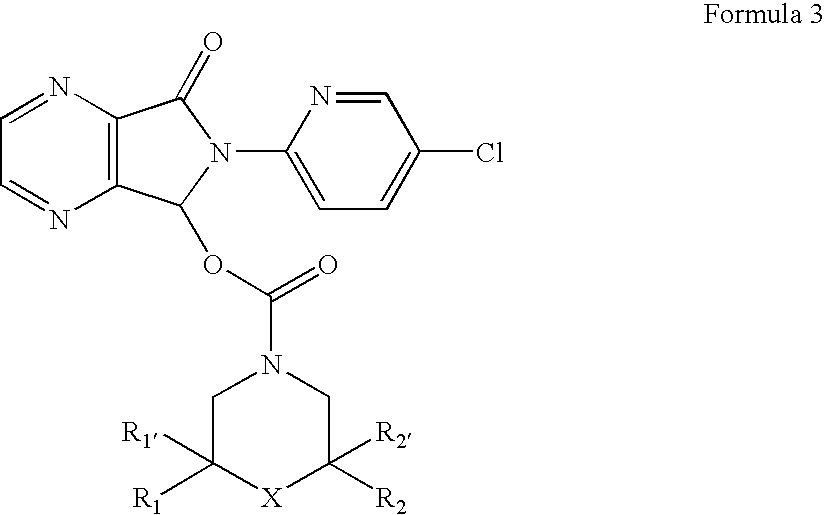
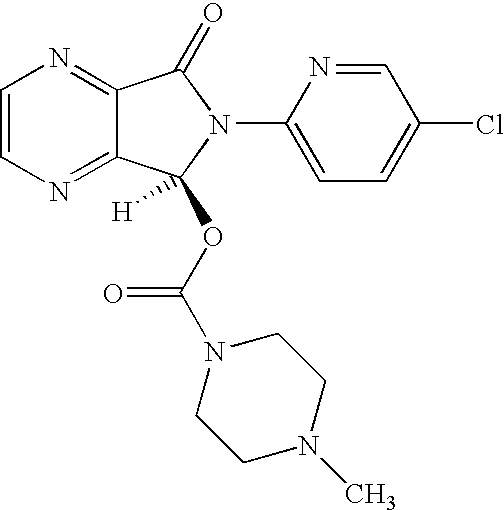
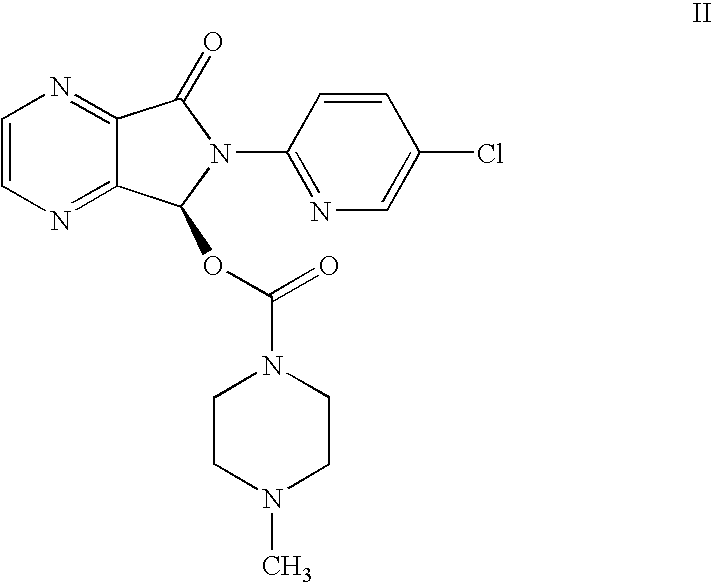
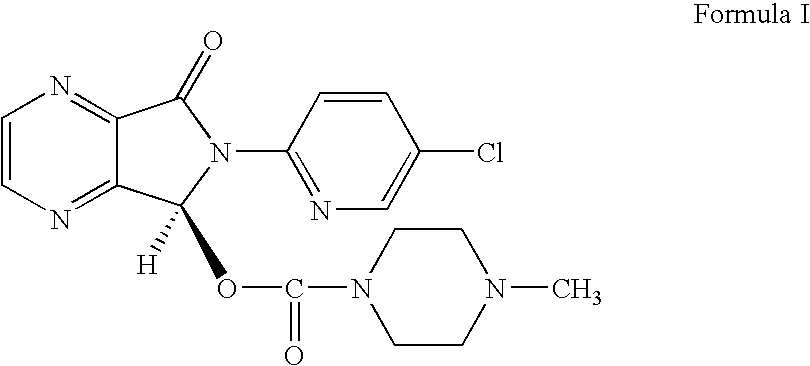
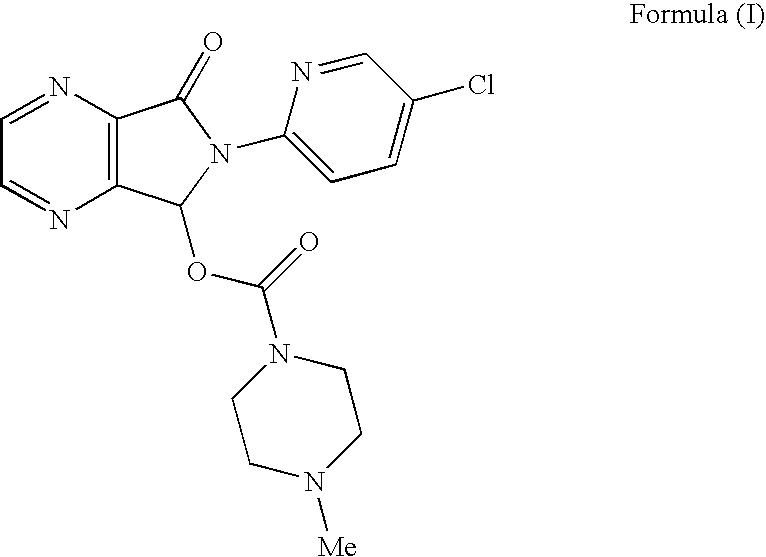
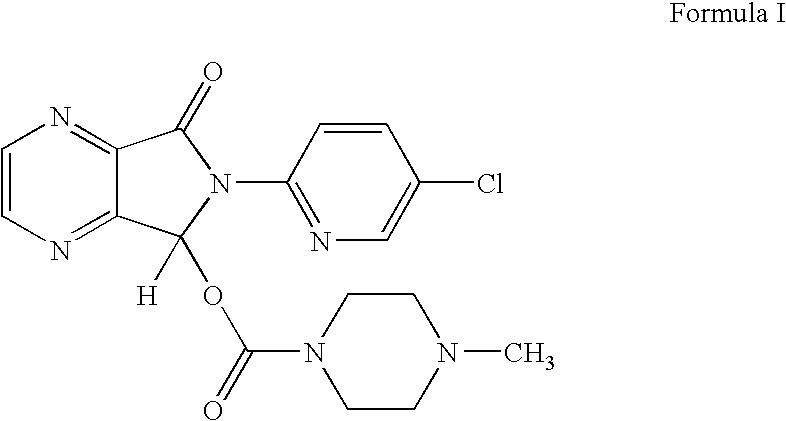
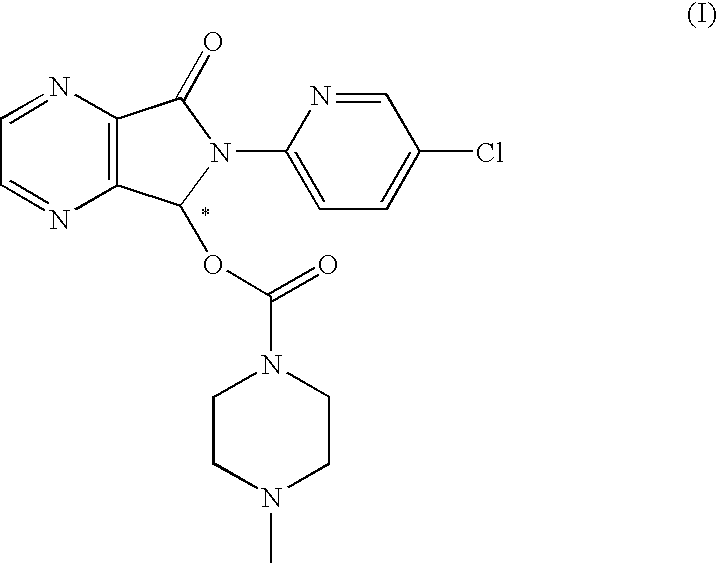
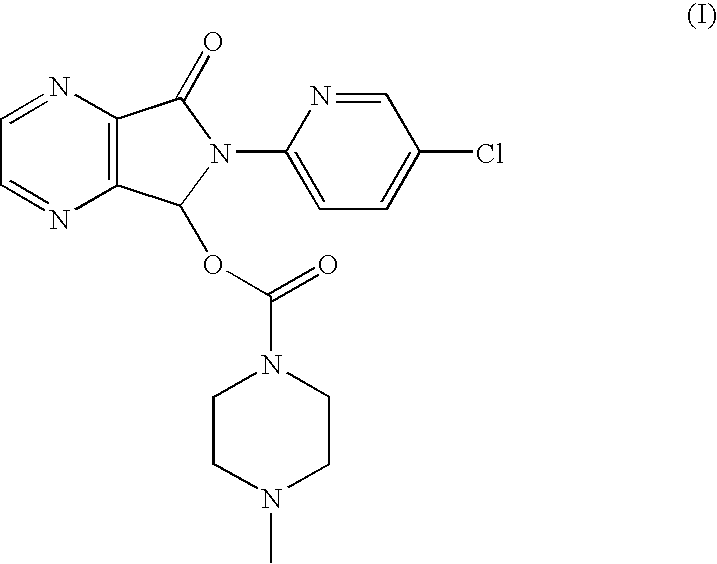
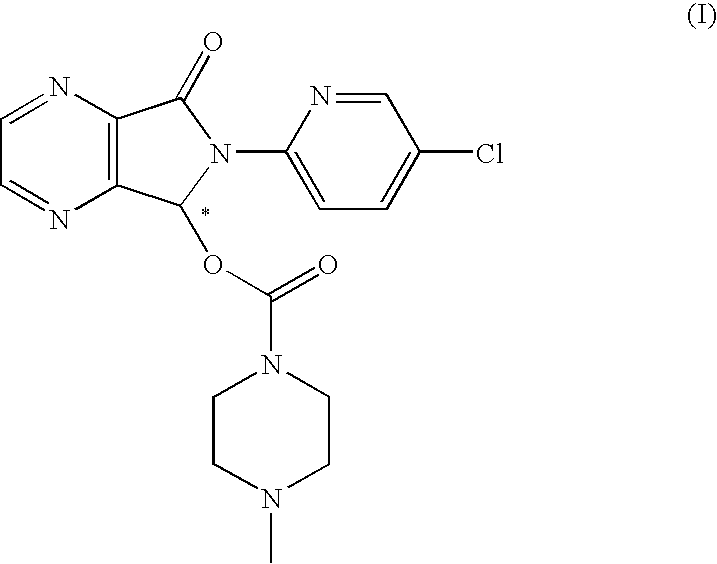
Process for the resolution of zopiclone and intermediate compounds
The present invention refers to a process for the resolution into one of its enantiomers of the racemate of compound of formula (I):which comprises separating said one of its enantiomers from a diastereoisomeric salt of formula (II), which is formed by reaction of the racemic mixture with an optically active acetylated amino acid of formula (III). The invention also refers to new intermediates which are useful to carry out the process of the invention.
Owner:ESTEVE QUIMICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com