Sedative-hypnotic preparation, compound preparation, preparation method and pharmaceutical composition thereof
A compound preparation and composition technology, applied in the field of pharmaceutical preparations, can solve problems such as excessive dust and poor stability
Active Publication Date: 2014-07-16
SHANGHAI ZHONGXI PHARMA
View PDF8 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0010] The technical problem to be solved by the present invention is to provide a sedative and hypnotic preparation, in order to overcome the poor stability of zopiclone and eszopiclone preparations in the prior art, and the defects of excessive dust in the preparation process. Preparation method and pharmaceutical composition thereof
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
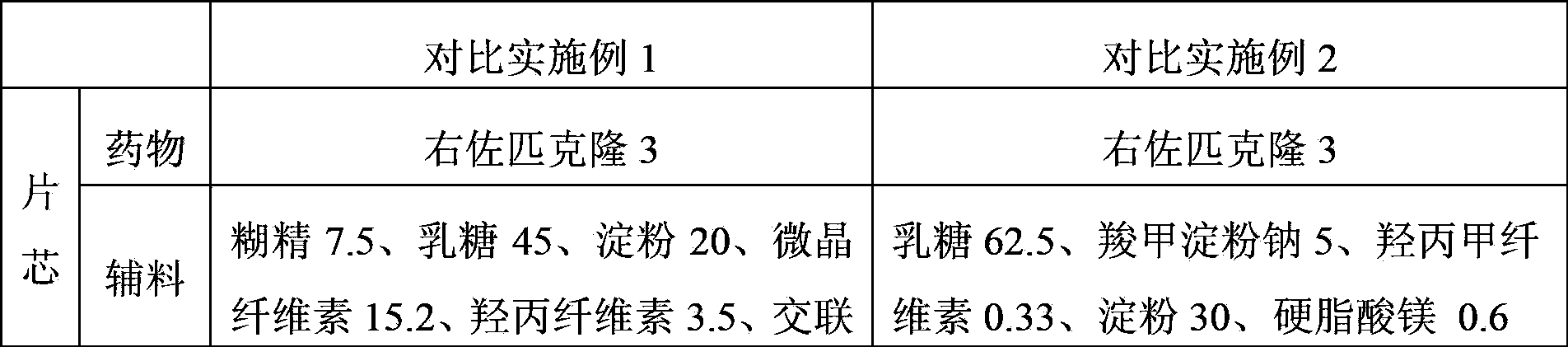
Embodiment 1 and Embodiment 2
[0070] Example 1 and Example 2 Eszopiclone tablets (3mg / tablet) (unit: gram)
[0071]
[0072]
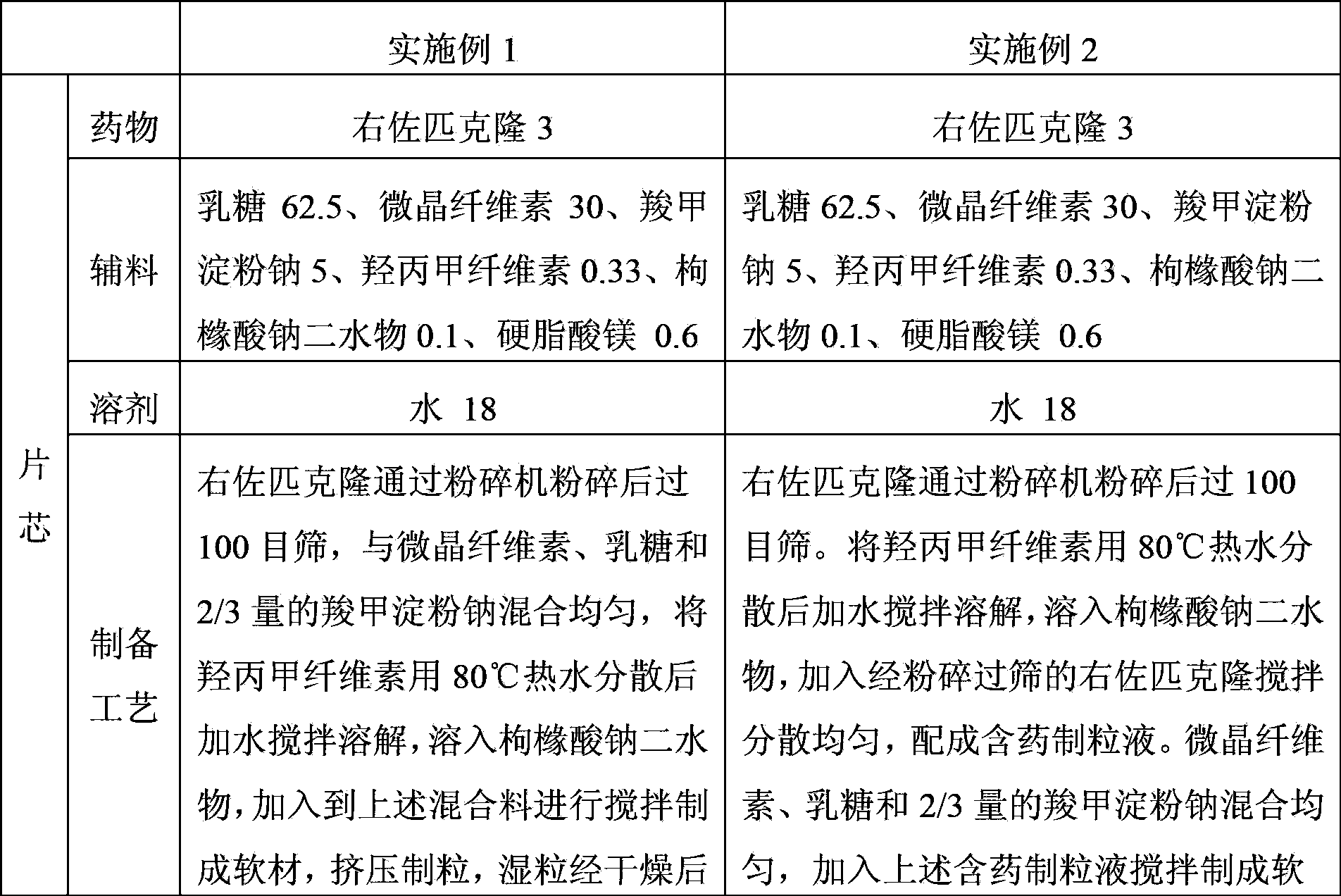
Embodiment 3
[0073] Example 3 (1mg / tablet) and Example 4 (3mg / tablet) eszopiclone tablets (unit: gram)
[0074]
[0075]
Embodiment 5 and Embodiment 6
[0076] Example 5 and Example 6 Eszopiclone Tablets (3 mg / tablet) (unit: gram)
[0077]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a sedative-hypnotic preparation, a compound preparation, a preparation method and a pharmaceutical composition thereof. The compound preparation of the sedative-hypnotic preparation includes zopiclone or eszopiclone and a pharmaceutically acceptable auxiliary material which includes a stabilizing agent with a dosage being, by mass, 0.1%-10% of the zopiclone or the eszopiclone. The stabilizing agent is one or more of a pharmaceutically acceptable organic acid salt, a pharmaceutically acceptable organic acid buffer pair and a pharmaceutical acceptable anti-oxidant. The pharmaceutical composition of the sedative-hypnotic preparation forms a compound preparation pharmaceutical composition with other pharmaceutical active substances. The preparation method of the sedative-hypnotic preparation or the compound preparation includes carrying out a wet granulation process to the pharmaceutical composition of the sedative-hypnotic preparation or the compound preparation pharmaceutical composition. The sedative-hypnotic preparation or the compound preparation is good in stability and is excellent in dissolution rate and content uniformity. Quality of the preparations can be ensured and the preparations are suitable for industrial production.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, and relates to a sedative and hypnotic preparation, a compound preparation thereof, a preparation method and a pharmaceutical composition thereof, in particular to a zopiclone or eszopiclone preparation, a compound preparation thereof, a preparation method and a pharmaceutical composition thereof thing. Background technique [0002] Eszopiclone (Eszopiclone) is a single isomer of zopiclone (zopiclone), a rapid short-acting non-benzodiazepine sedative hypnotics developed by Sepracor Corporation of the United States. Eszopiclone was launched in the United States in 2005. At that time, more than 2,000 people were involved in clinical trials for the treatment of short-term and chronic insomnia. Preclinical and clinical studies showed that the affinity of eszopiclone for benzodiazepine receptors is 50 times of eszopiclone, LD of eszopiclone 50 It is 1500mg / kg, the L-body is 300mg / kg, and t...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/4985A61K31/381A61K31/335A61K31/138A61K31/135A61K9/16A61P25/20
Inventor 郑斯骥张琦谭波阮圆
Owner SHANGHAI ZHONGXI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com