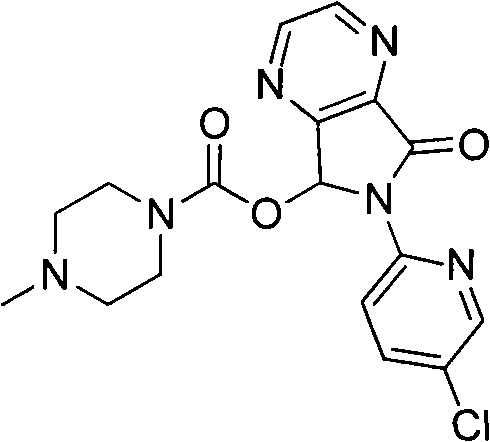
Preparation method of zopiclone
A technology of zopiclone and compounds, applied in the field of preparation of zopiclone, can solve the problems of less than 80% total yield, skin burns, unsafety, etc., achieve production conditions and environmental friendliness, reduce the discharge of three wastes, The effect of simplifying operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
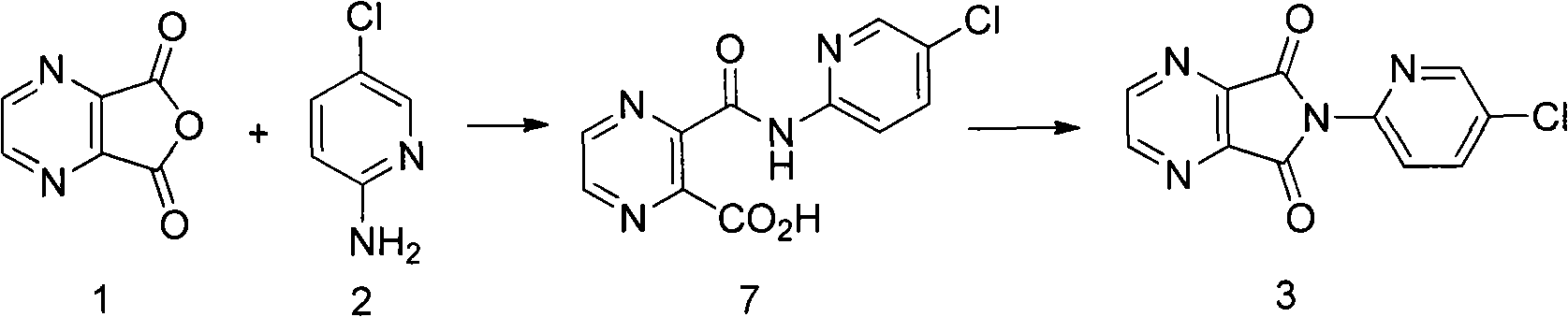
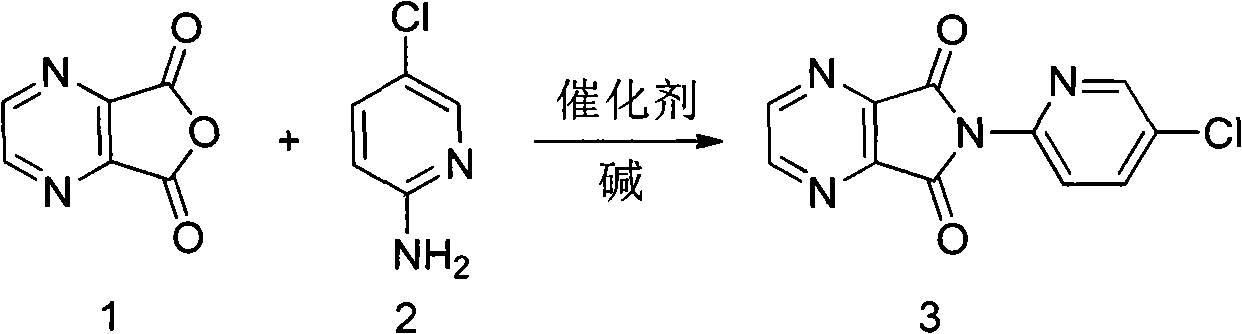
[0025] Embodiment 1: the preparation of compound 3
[0026] Add 15g of 2,3-pyrazine dicarboxylic anhydride, 13g of 2-amino-5-chloropyridine, 0.12g of 4-dimethylaminopyridine, and 120mL of xylene to a 250mL three-neck flask equipped with mechanical stirring and a spherical reflux tube. , 20.2g triethylamine was added at 30°C. Raise the temperature to 80°C and react for 8h; continue to raise the temperature to reflux and react for 1h. Stop heating, naturally cool down to room temperature under mechanical stirring, and continue stirring for 2 hours; use an ice bath to cool down, control the internal temperature at 0-5°C, and stir for 2 hours. After suction filtration, the filter cake was washed once with 50 mL of 1N dilute hydrochloric acid, washed again with 50 mL of water, and dried in a 60°C oven for 8 hours to obtain 22.1 g of an off-white solid with a yield of 85%, a purity of 98%, and a melting point of 232-235°C.
Embodiment 2
[0027] Embodiment 2: the preparation of compound 4
[0028] Add 50 g of compound 3 and 300 mL of dioxane into a 1000 mL three-necked flask equipped with a mechanical stirrer and a thermometer, and stir to dissolve the solid. Cool down in an ice-water bath, control the internal temperature at 10-15°C, add 3.7g of potassium borohydride in batches, after the addition is complete, continue the reaction for 0.5h, add 50mL of tap water and continue stirring for about 6h. The reaction solution was poured into 500 g of ice water, stirred for 2 h, and then suction-filtered to obtain 44 g of a light yellow solid with a yield of 87.4% and a purity of 98% by HPLC.
Embodiment 3
[0029] Embodiment 3: the preparation of zopiclone
[0030] In a 500mL three-neck flask equipped with mechanical stirring and a spherical reflux condenser, add 20g of compound 4, 18.6g of compound 5, 300mL of dichloromethane, 35mL of anhydrous triethylamine, 2.0g of 4-dimethylaminopyridine, and stir at room temperature for 2h Afterwards, the reaction was carried out under reflux for 1 h, and TLC showed that the reaction of the substrate was completed. Naturally cooled to room temperature, a solid precipitated, suction filtered, the filtrate was washed with 1N hydrochloric acid (50mL x 2), and the solvent was evaporated to dryness under reduced pressure to obtain an off-white solid, which weighed 26.7g after drying. The solid was dissolved in 250 mL of ethyl acetate under reflux, decolorized with 2 g of activated carbon at the same time, filtered while hot after 1 h, the filtrate was cooled and crystallized for 4 h, and filtered with suction to obtain 23.6 g of white solid with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com