Eszopiclone-containing particle and its preparation method
A technology for eszopiclone and granules, which is applied in the field of eszopiclone-containing granules and their preparation, can solve the problems of gastrointestinal adverse reactions, complicated composition and preparation methods, and restrictions on the development of eszopiclone oral preparations, etc. The advantages of drug application: no bad smell, avoid gastrointestinal adverse reactions, and overcome the effect of difficult preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 3
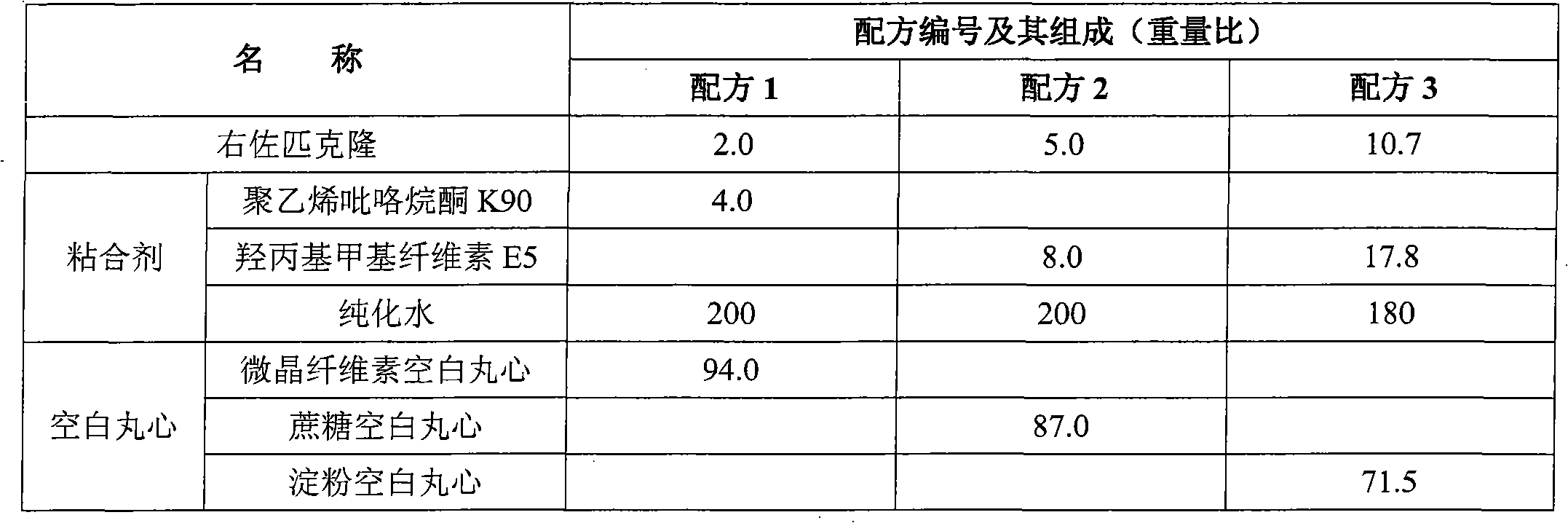
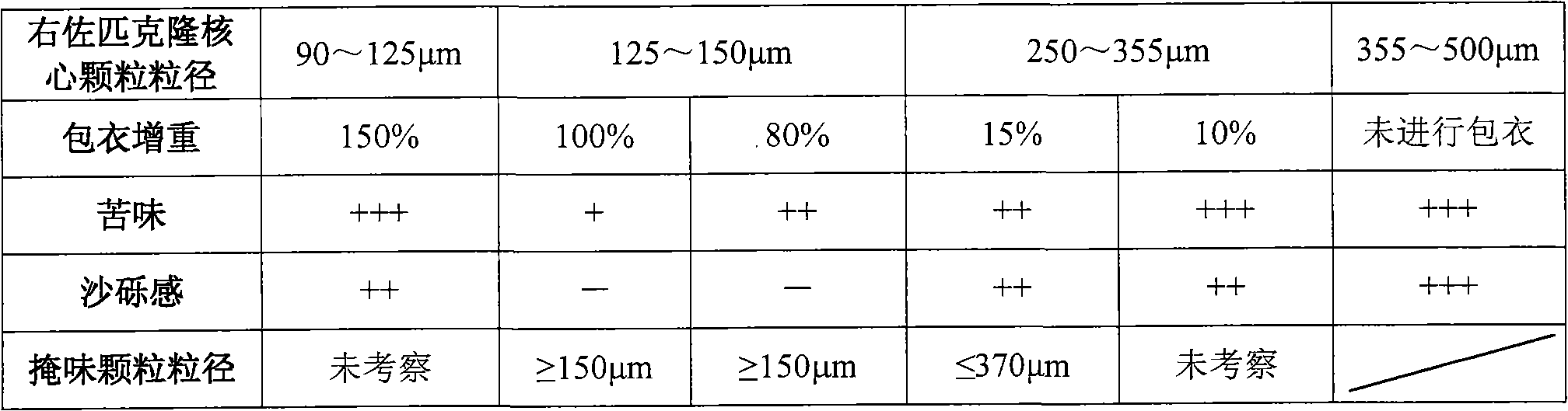
[0098] Embodiment formula and evaluation result are shown in Table 10, Table 11, Table 12.
[0099] Preparation method: Dissolve the adhesive in water, add eszopiclone powder (through a 200-mesh sieve), and disperse for 15 minutes under high shear (IKAT25 high-shear disperser, 1000 rpm) to make a suspension; take the blank The pellet core is placed in a fluidized bed, heated by fluidization, and the suspension containing eszopiclone is sprayed on the blank pellet core by the bottom spray method, and the fluidized drying is continued to obtain the drug-loaded core particle; the surfactant and / or Disperse the plasticizer with water, slowly add the film-forming material under stirring to obtain a water dispersion, take the anti-sticking agent and add it to the water for high-shear dispersion for 15 minutes (IKA T25 high-shear disperser, 1000 rpm), and then add it to the aforementioned water dispersion The coating solution is obtained in the body; the above-mentioned drug-loaded c...
Embodiment 4 to 6
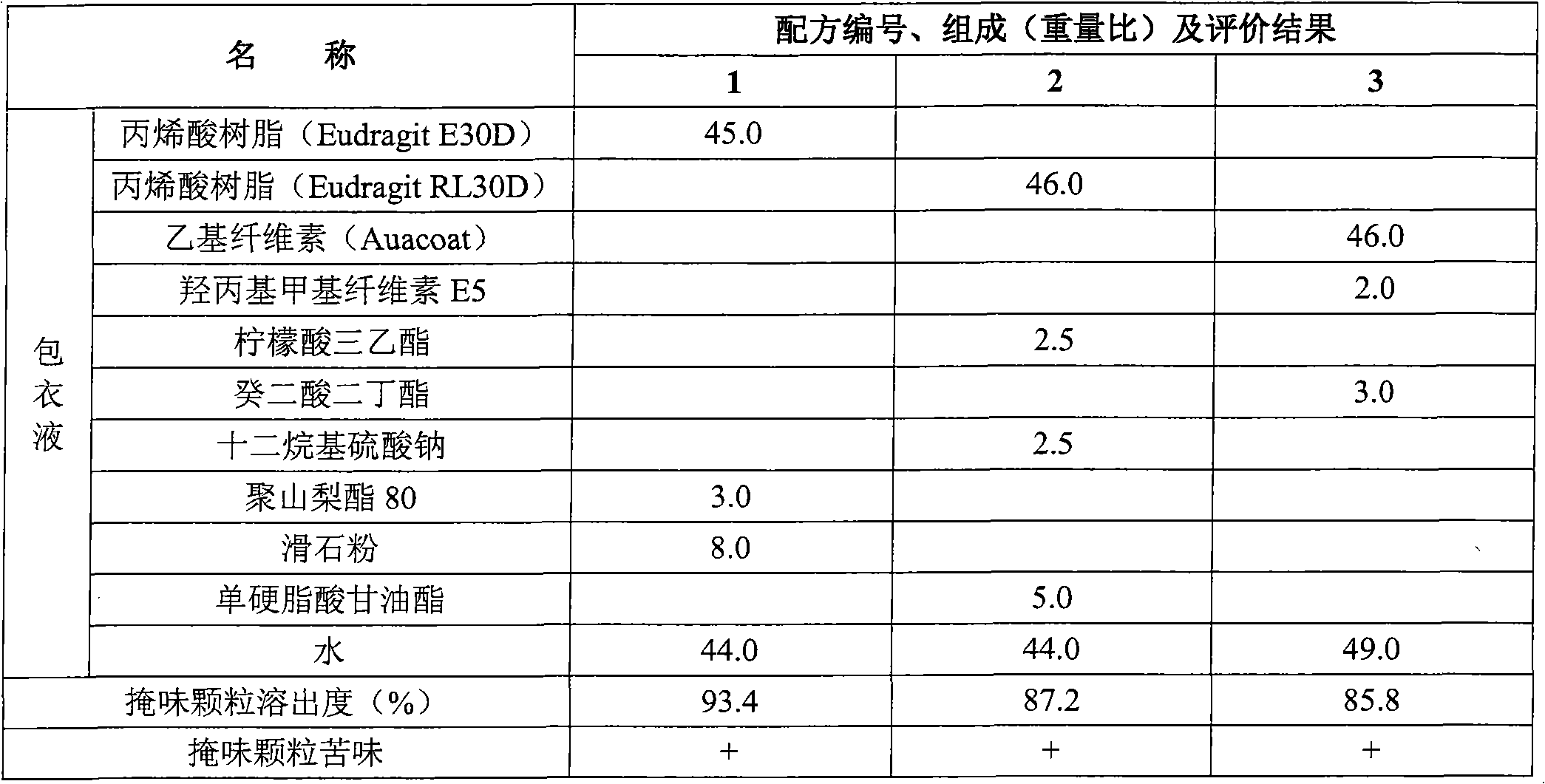
[0109] Embodiment formula and evaluation result are shown in Table 13, Table 14, Table 15.
[0110] Preparation method: Dissolve the binder in water, add eszopiclone powder (passed through a 200-mesh sieve), and disperse for 15 minutes under high shear (IKA T25 high-shear disperser, 1000 rpm) to make a suspension; The blank core is placed in a fluidized bed, heated by fluidization, and the suspension containing eszopiclone is sprayed onto the blank core by the bottom spray method, and the fluidized drying is continued to obtain drug-loaded core particles; film-forming materials, plasticizers Agent, anti-sticking agent are dispersed with ethanol, high-shear dispersion 15 minutes (IKA T25 high-shear disperser, 1000 revs / min), obtain taste-masking coating liquid; Taste-masked granules are obtained by coating.
[0111] Table 13 Example 4 to 6 Eszopiclone core granule formula (by weight ratio)
[0112]
[0113] Table 14 embodiment 4 to 6 coating solution formula (by weight rat...
Embodiment 7
[0118] Example 7 (preparation of orally disintegrating tablets using eszopiclone taste-masking granules)
[0119] Take the eszopiclone taste-masking granules prepared in Example 5, mix them evenly with the excipients except magnesium stearate according to the formula in Table 16, then add magnesium stearate and mix for 5 minutes, and press into tablets to adjust the tablet weight to about 200 mg.
[0120] Table 16 Eszopiclone taste-masked orally disintegrating tablet and its evaluation results (by weight ratio)
[0121] Material name
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com