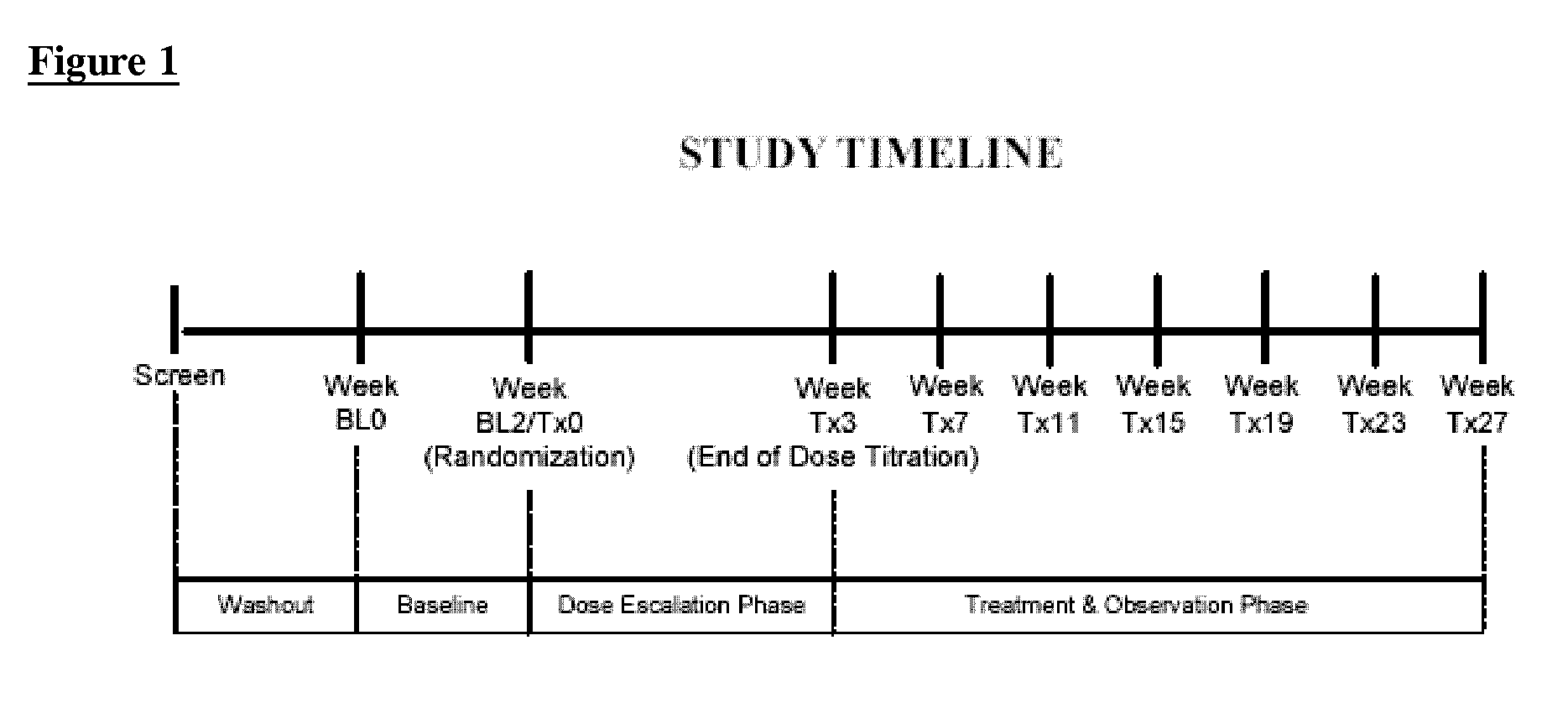
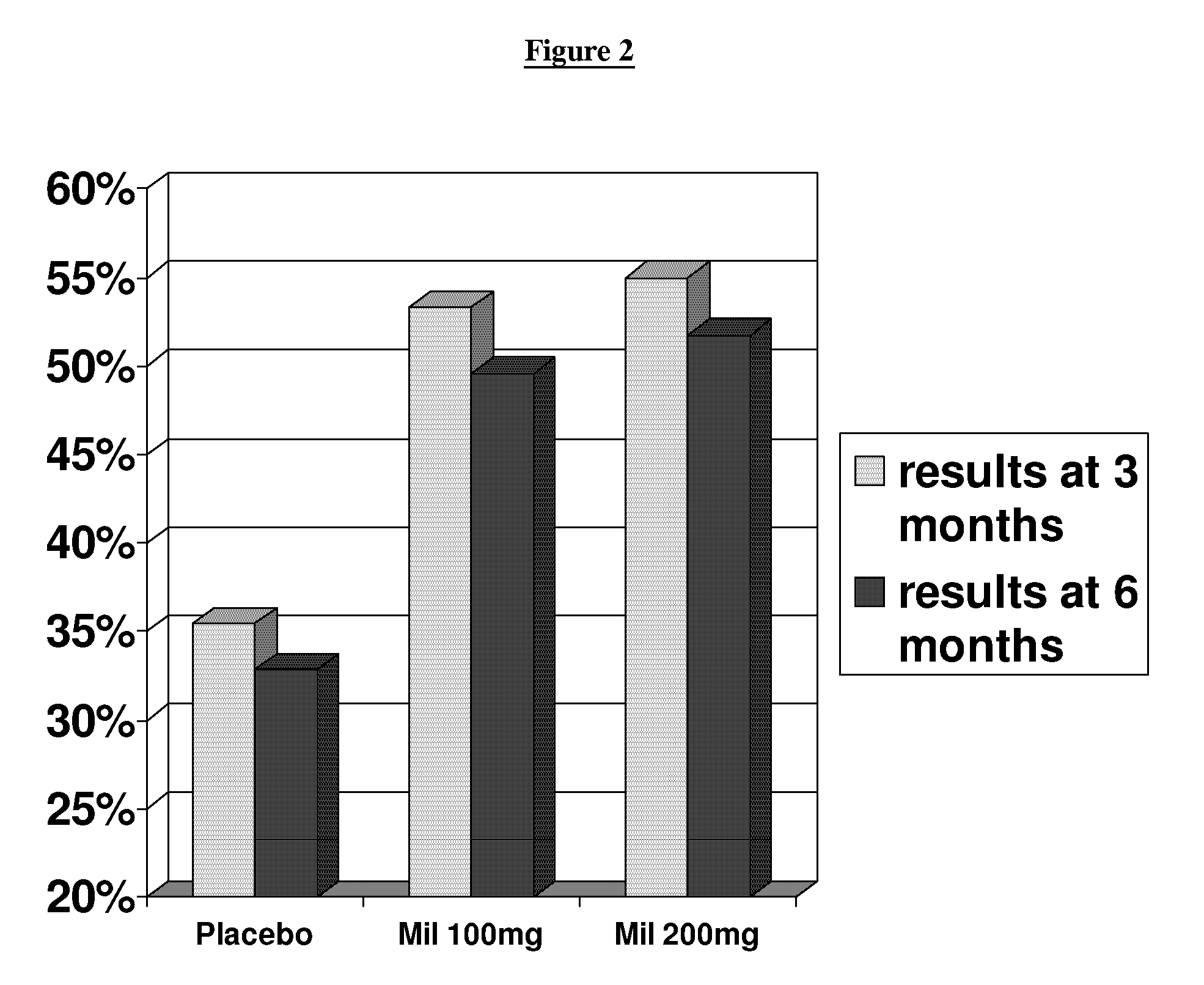
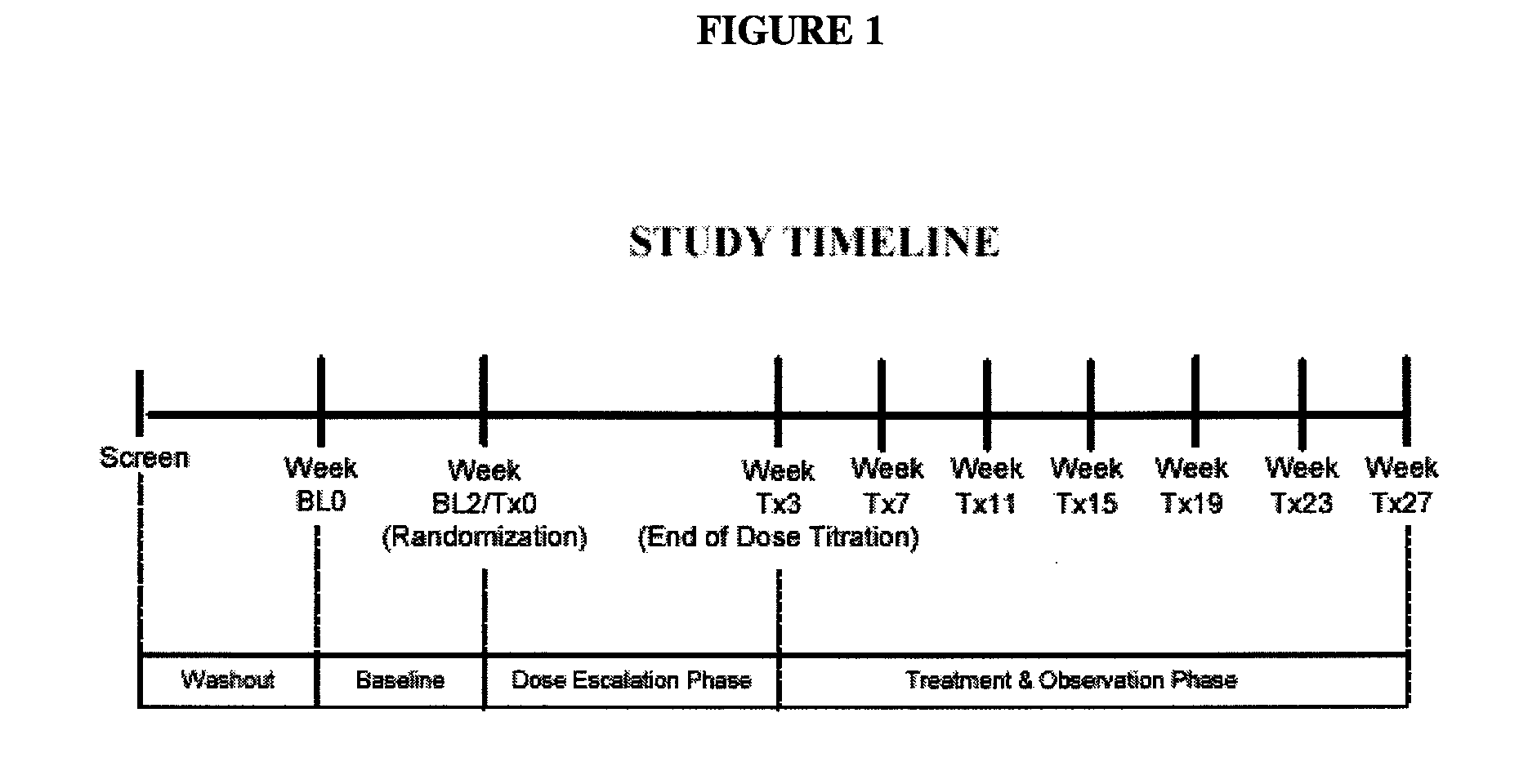
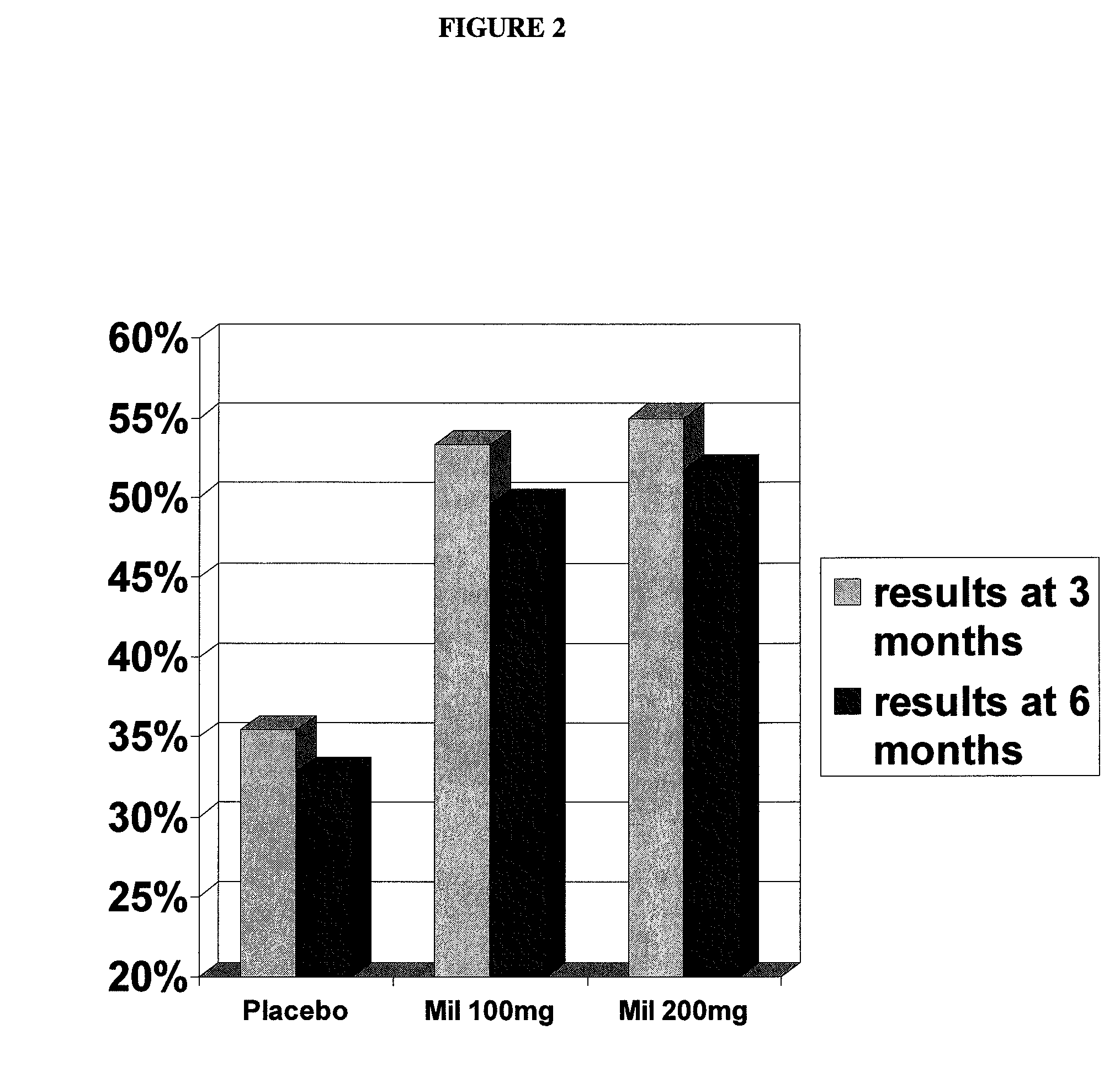
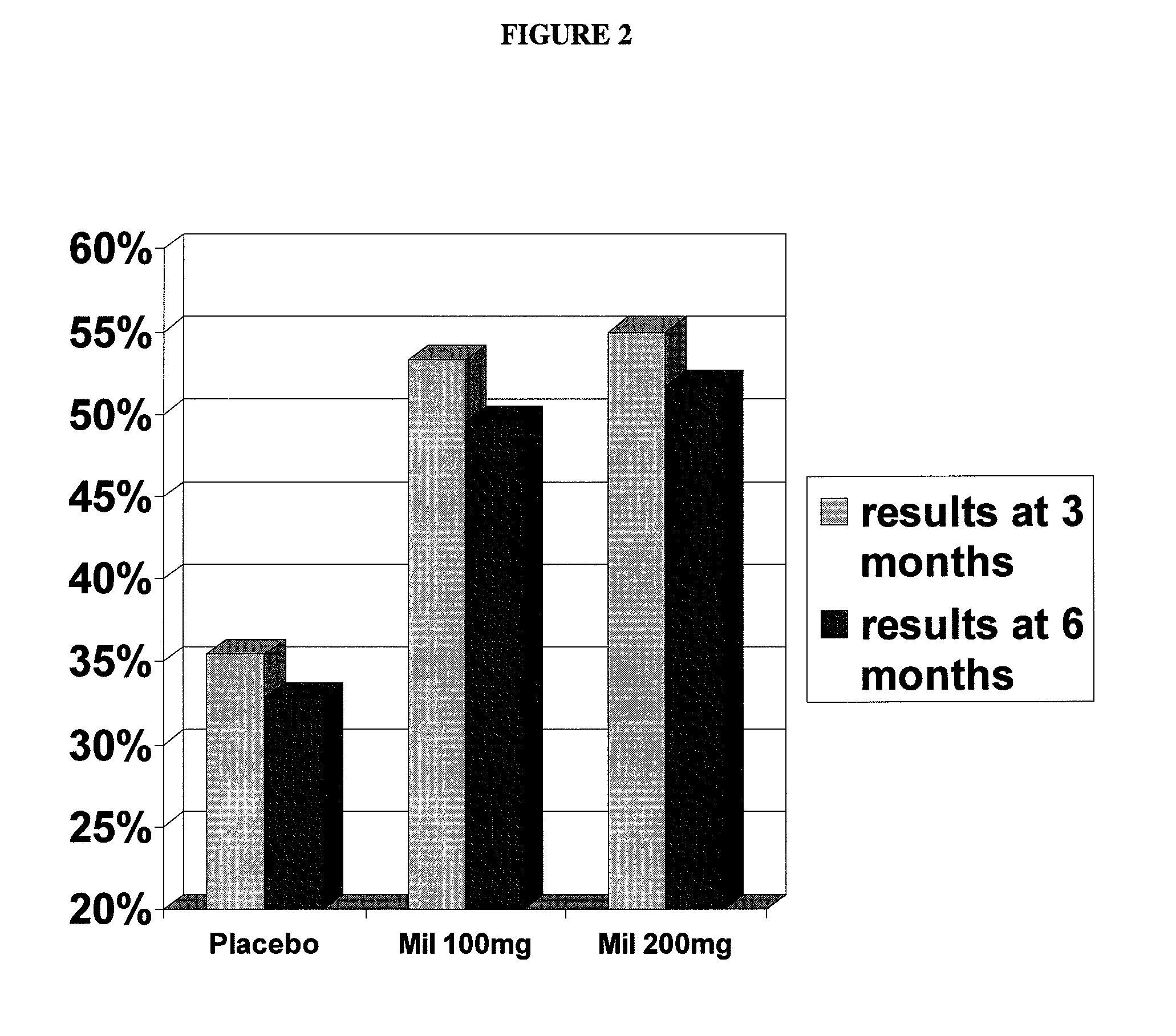
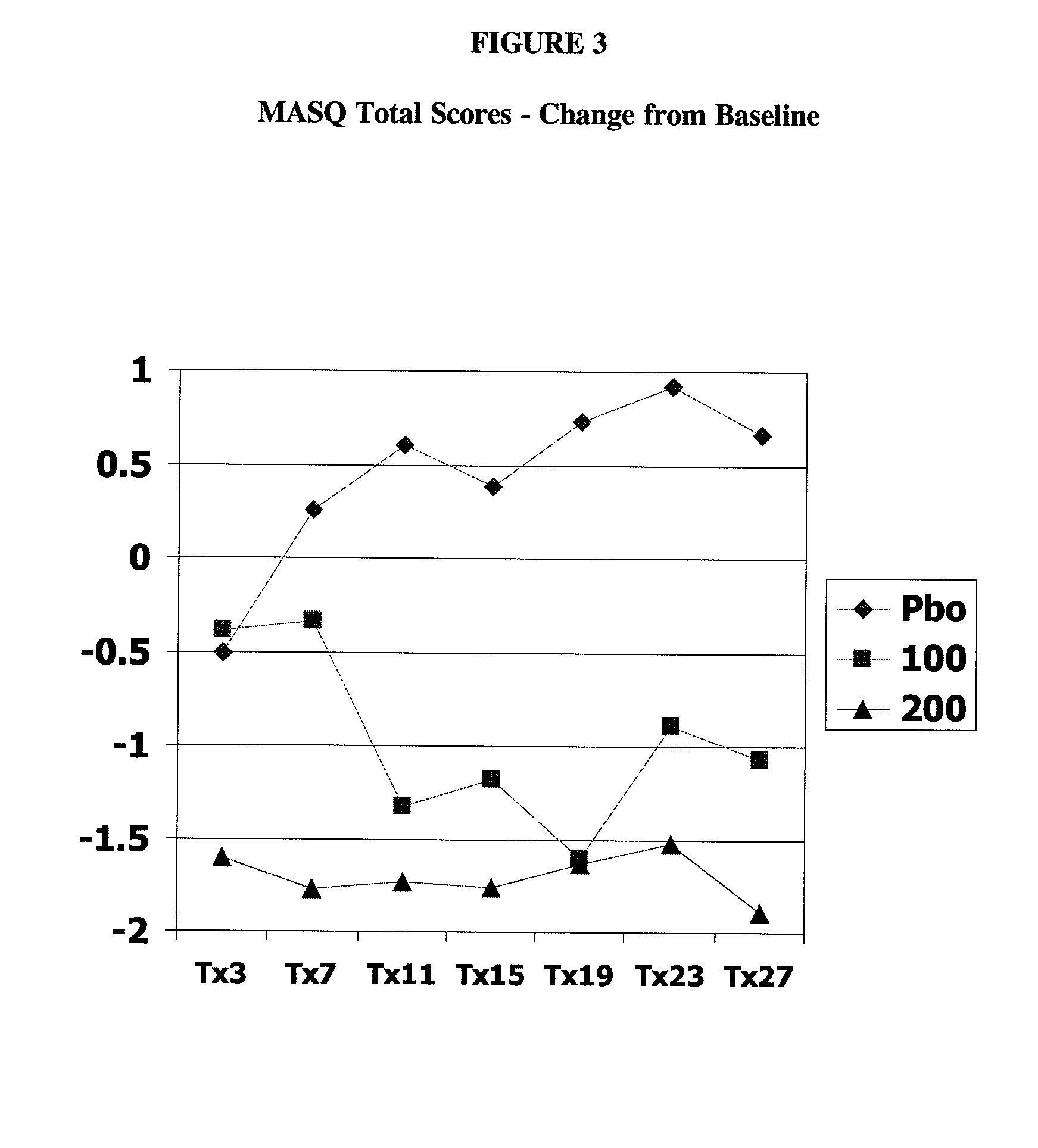
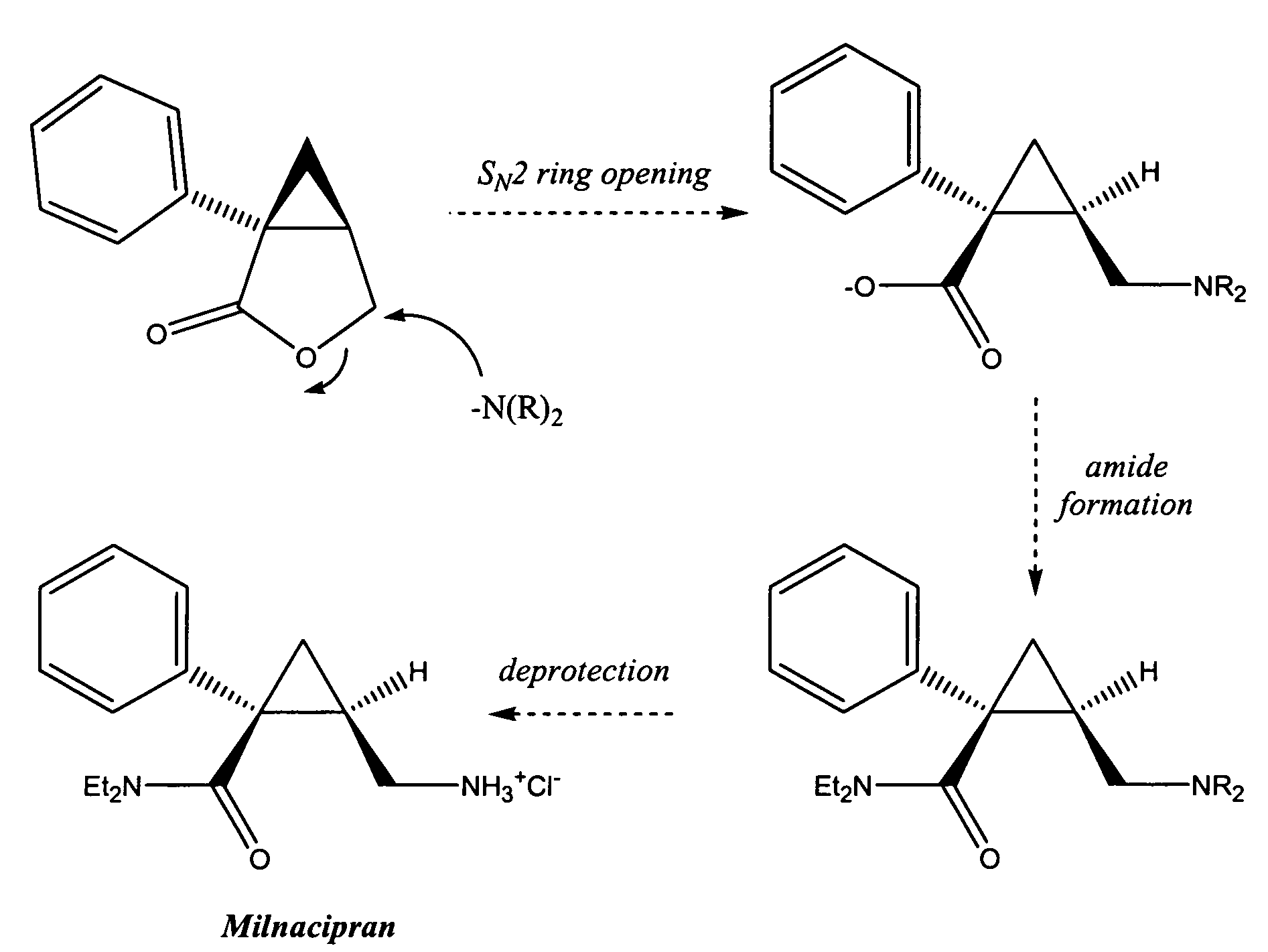
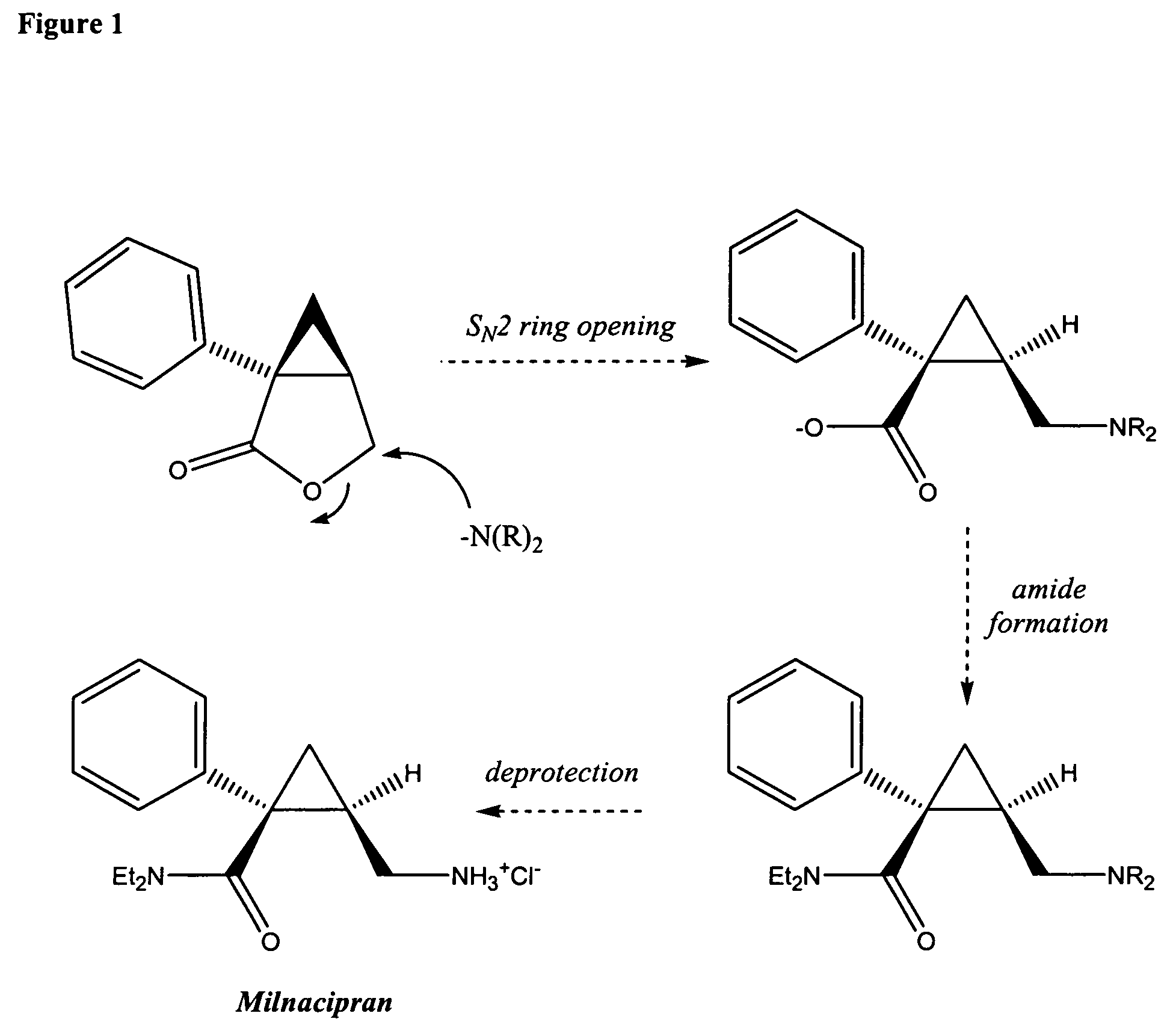
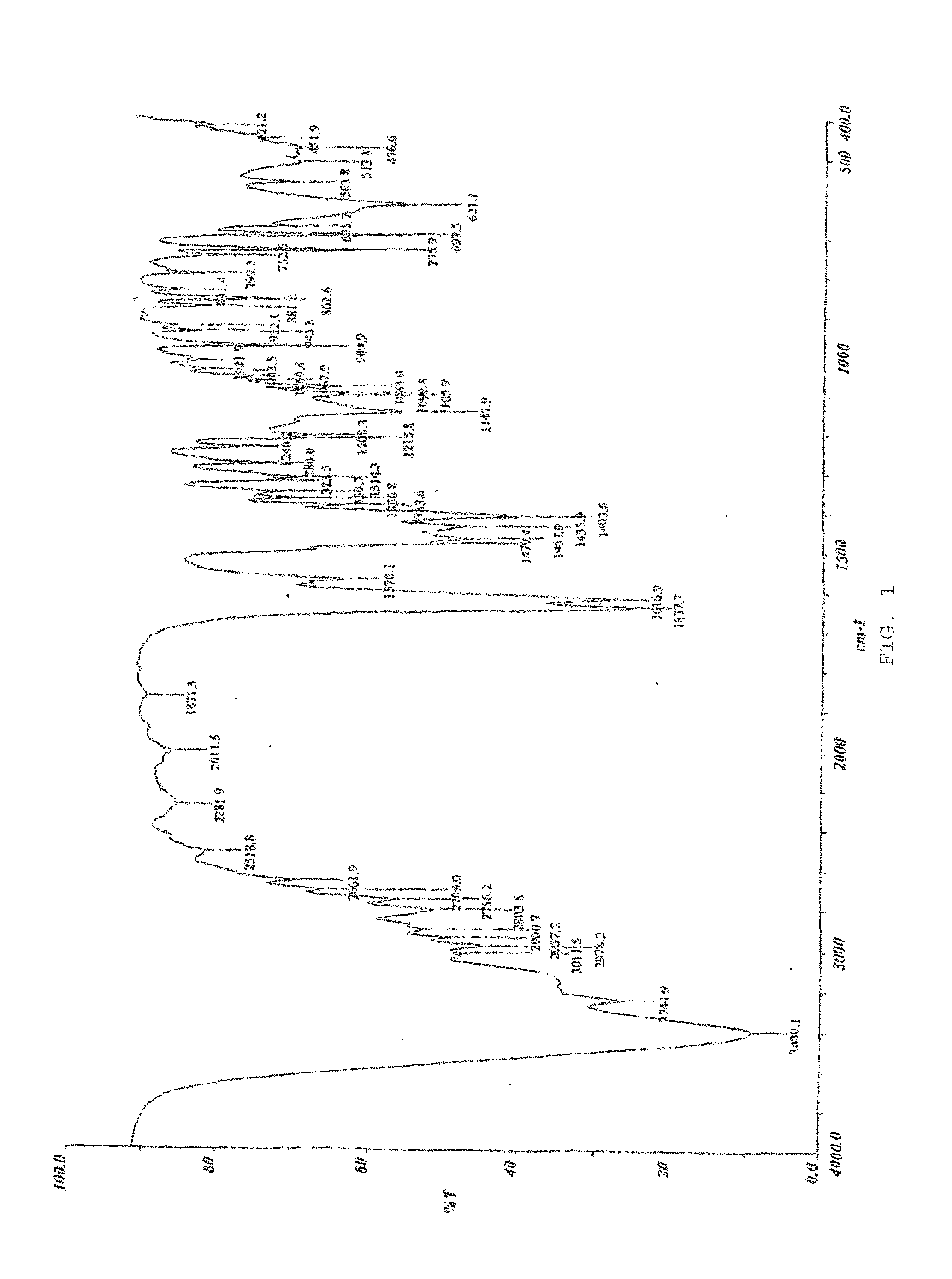
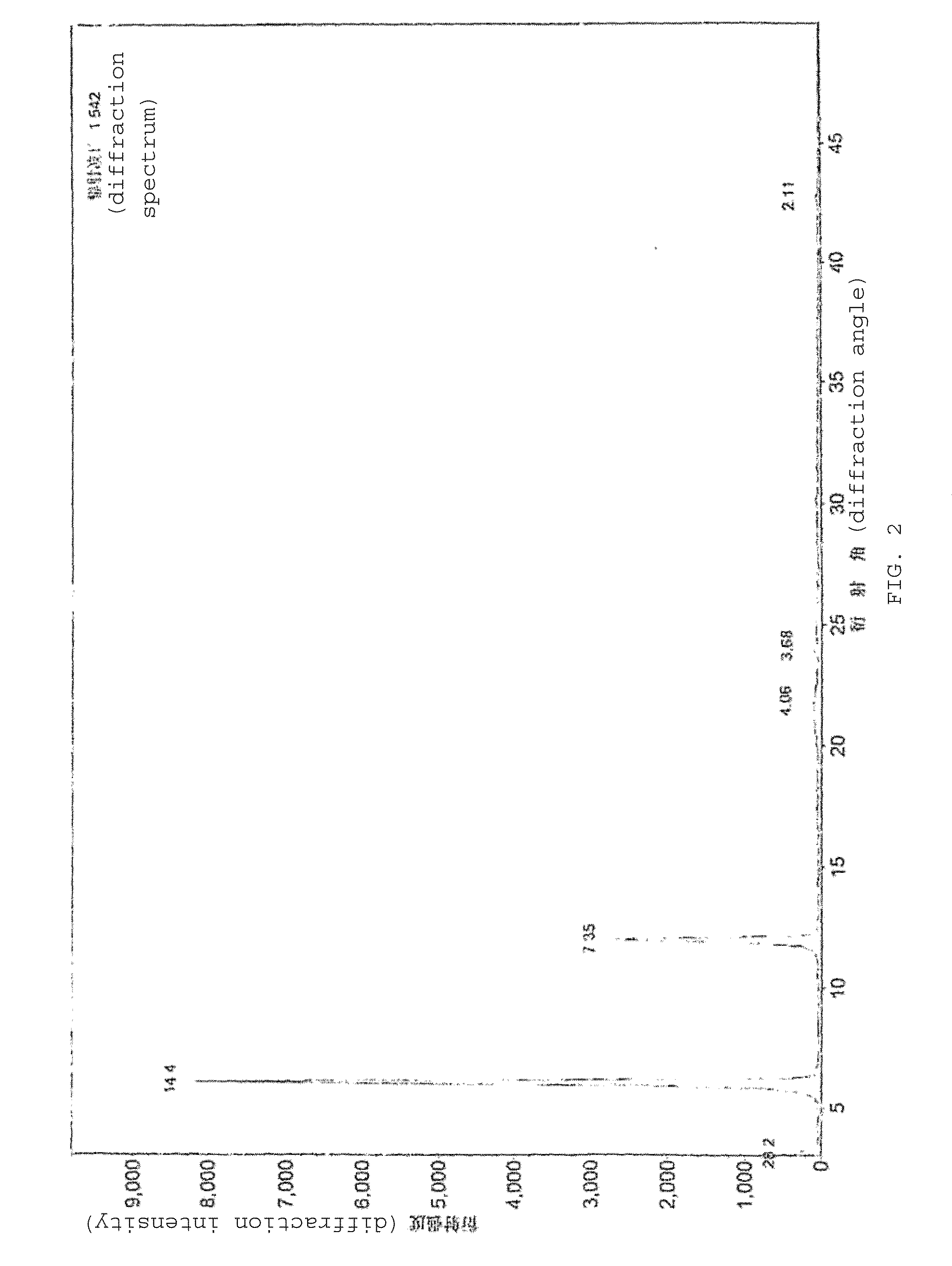
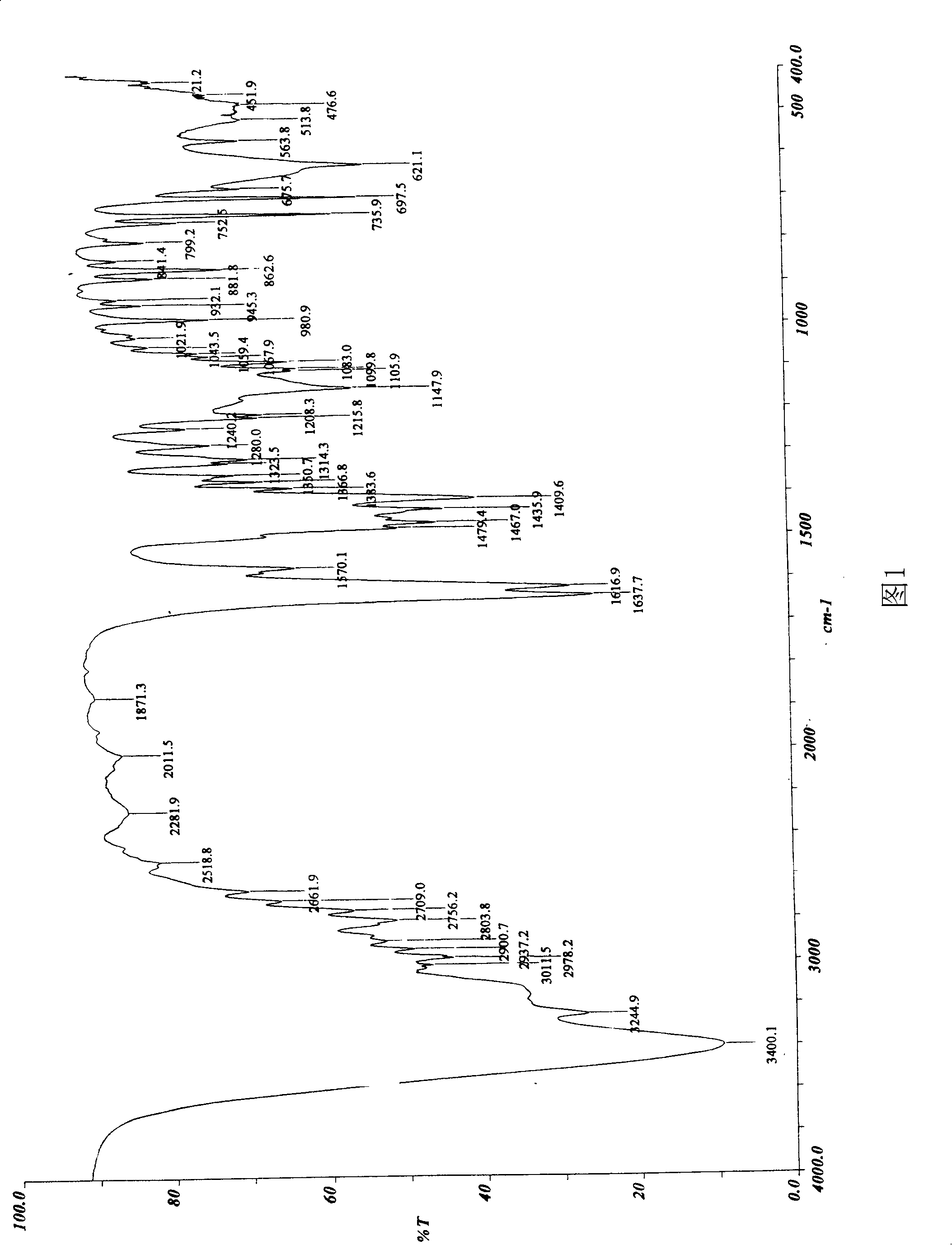
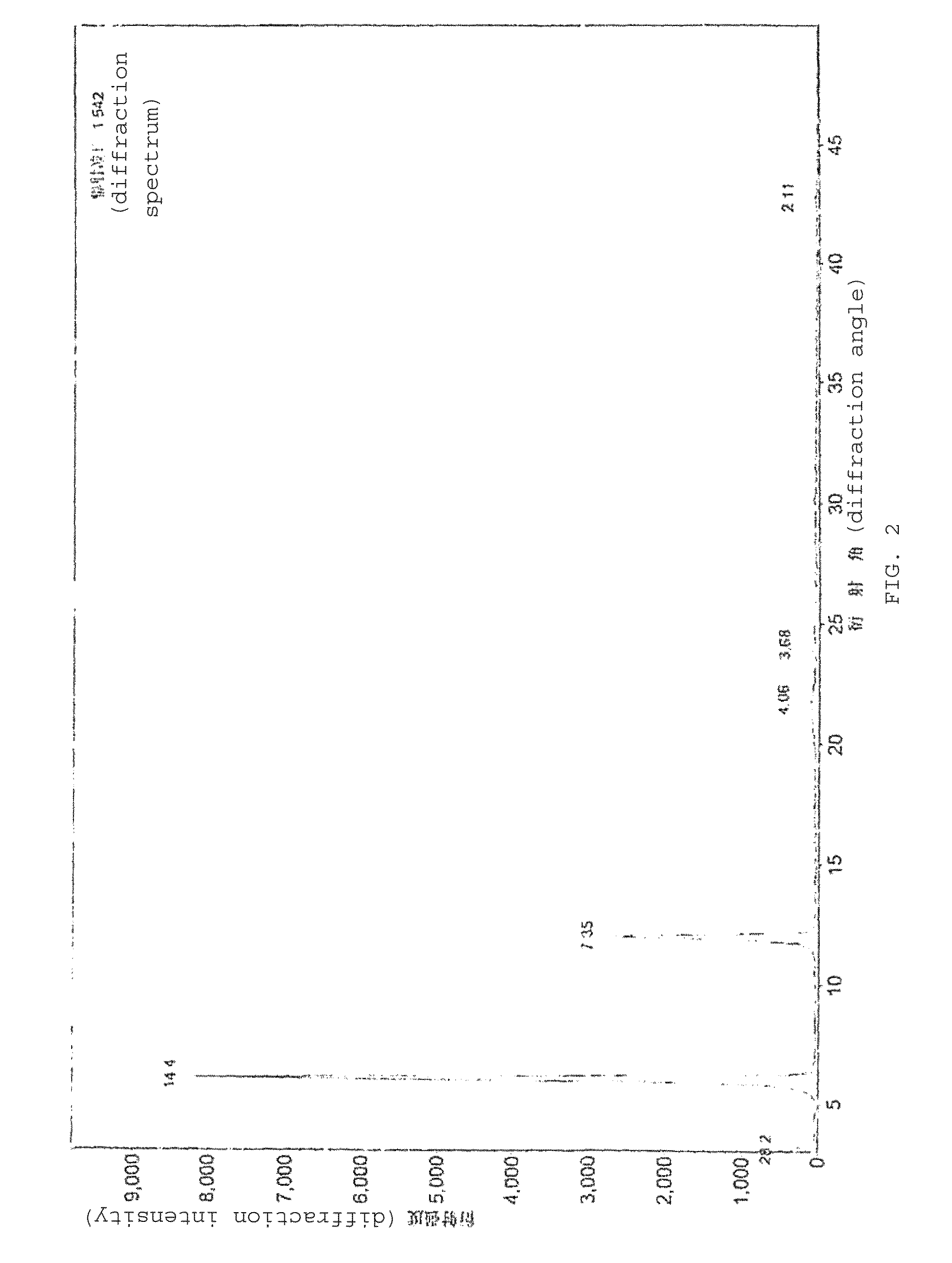
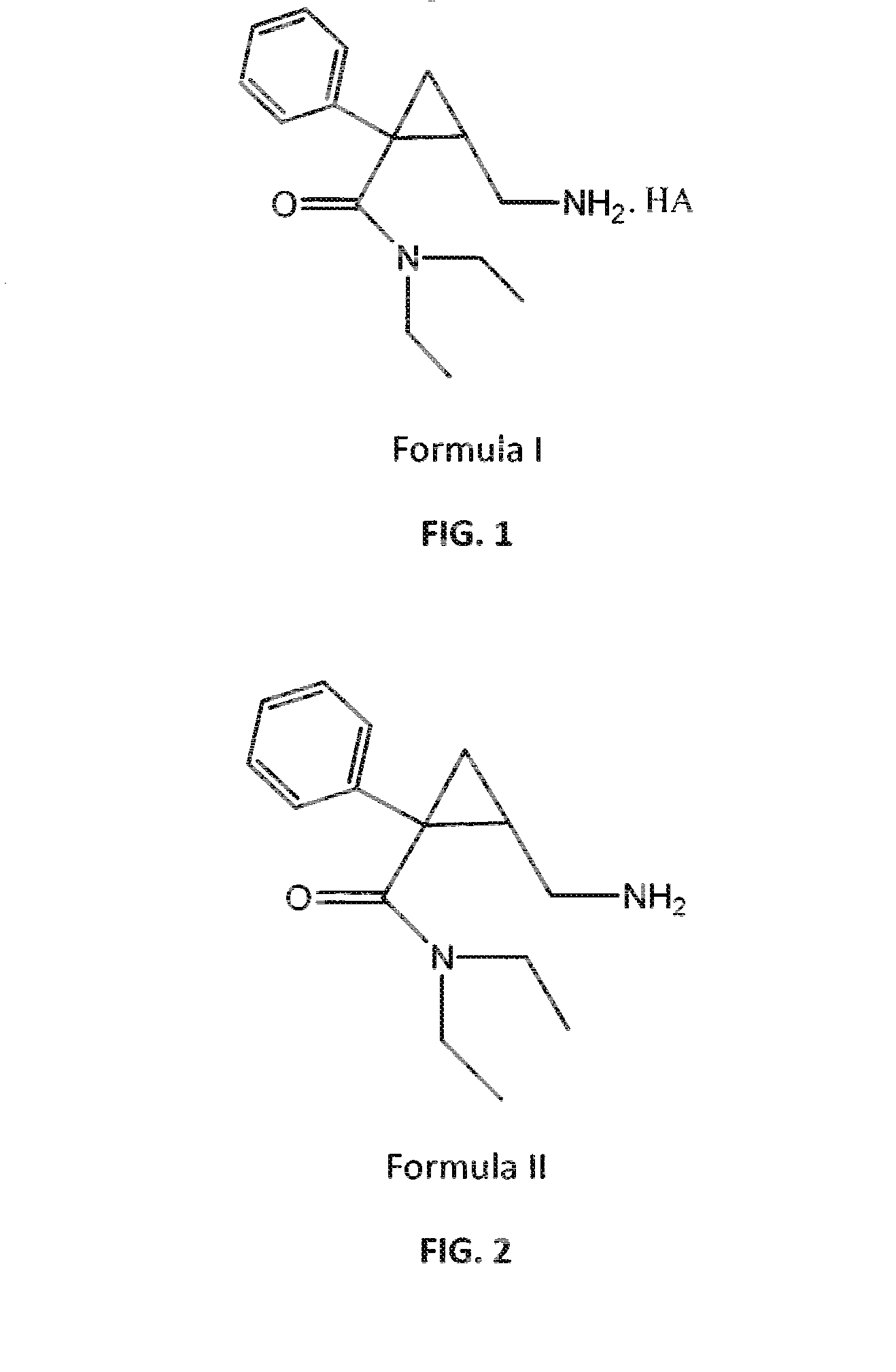
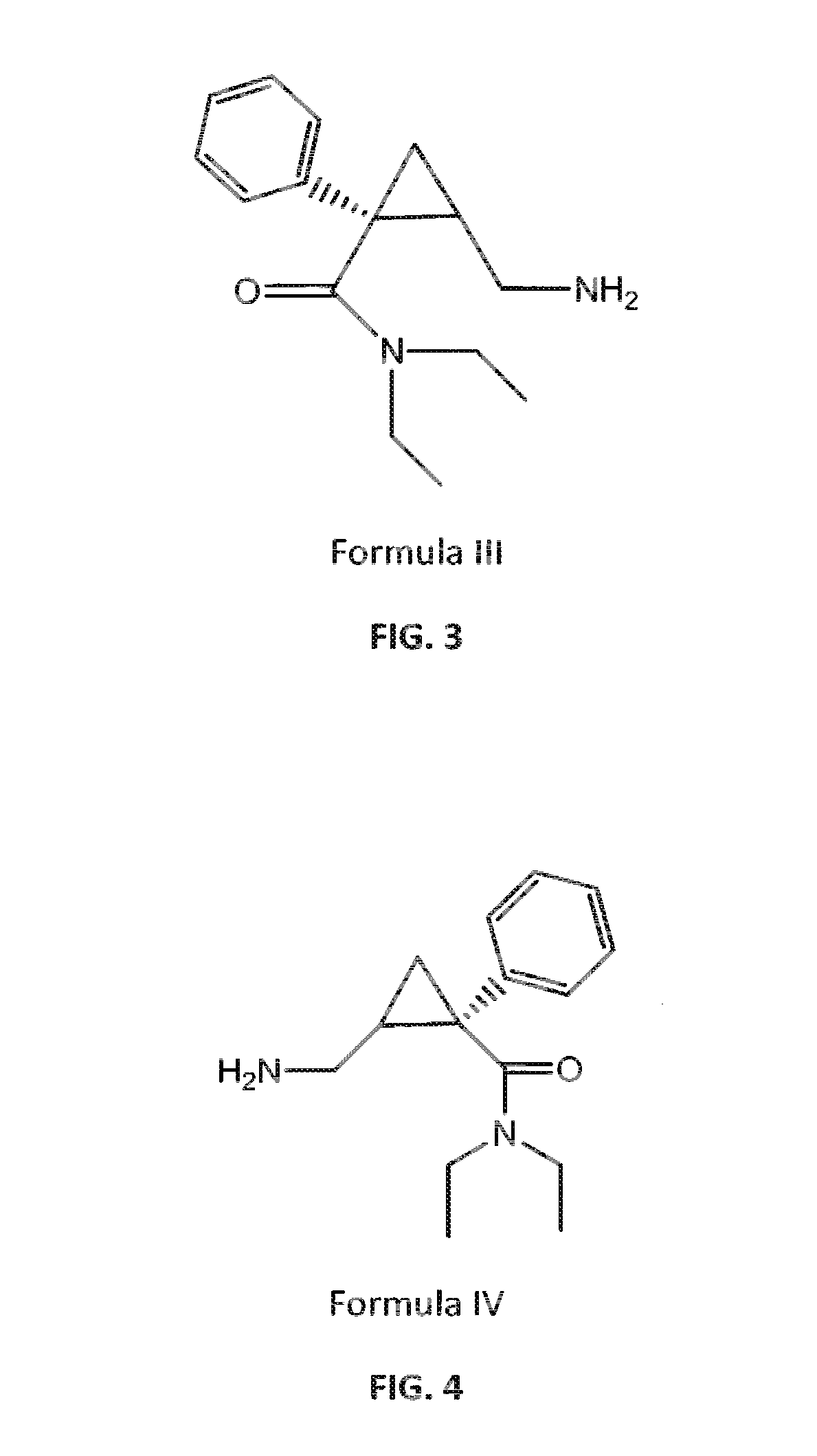
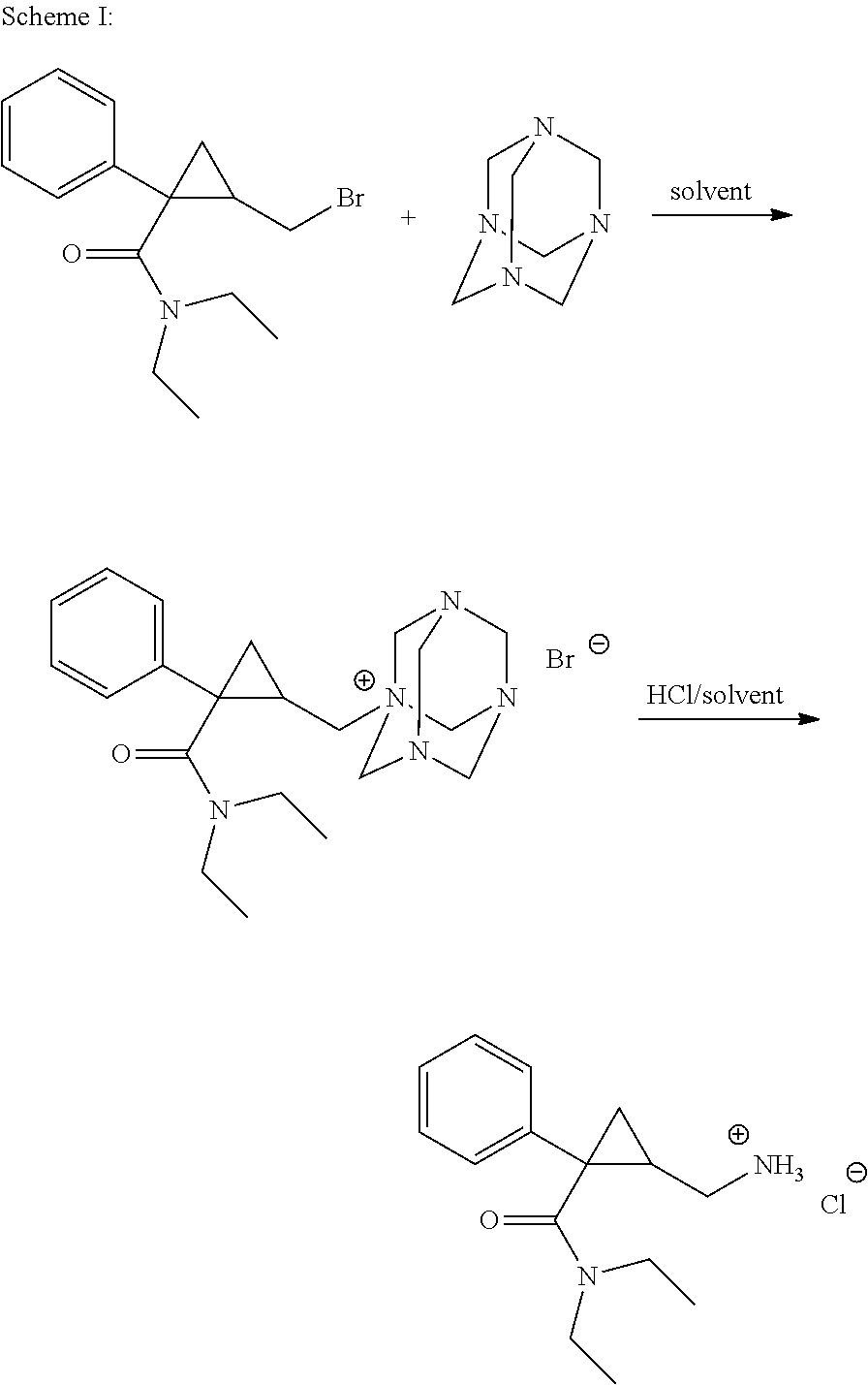
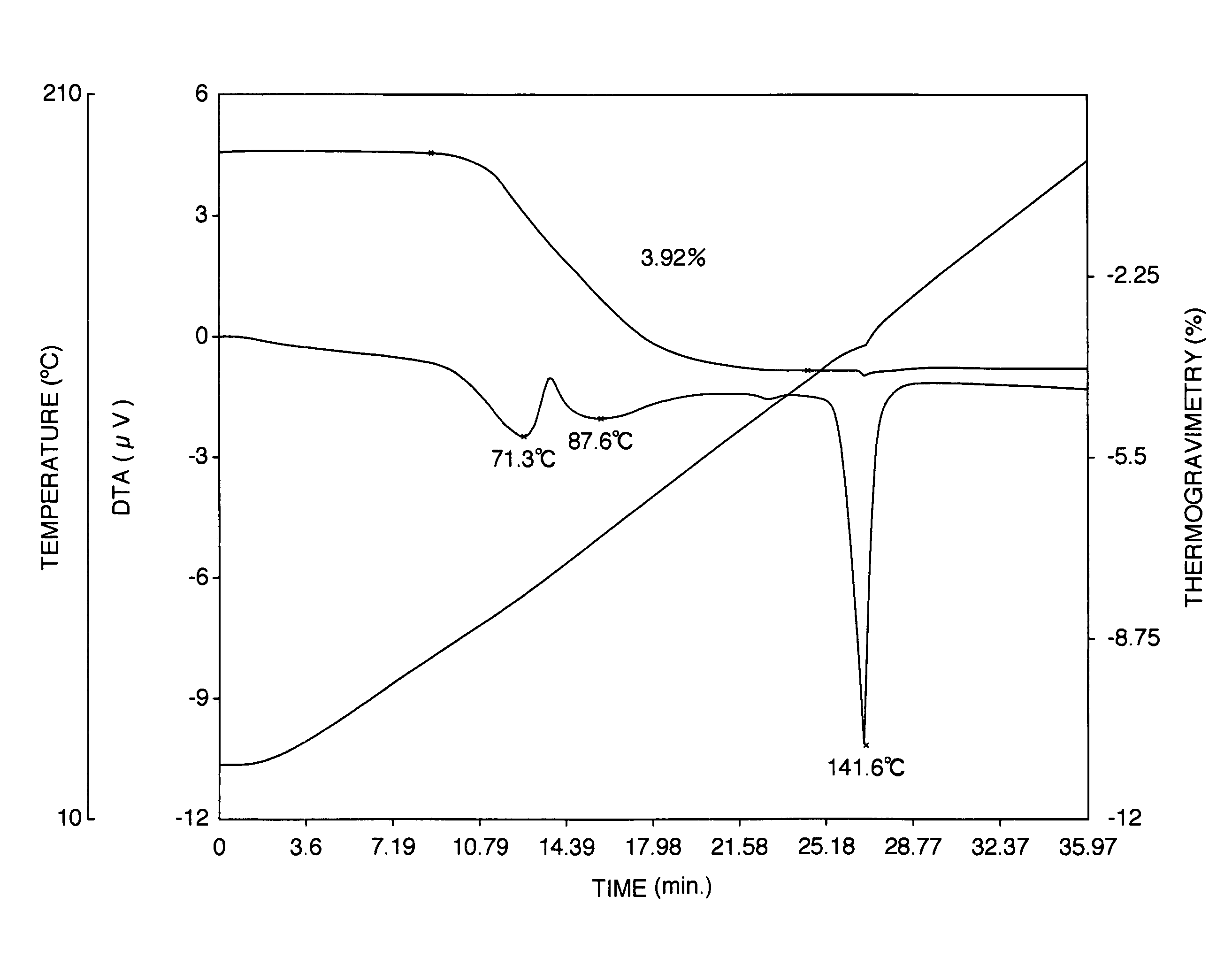
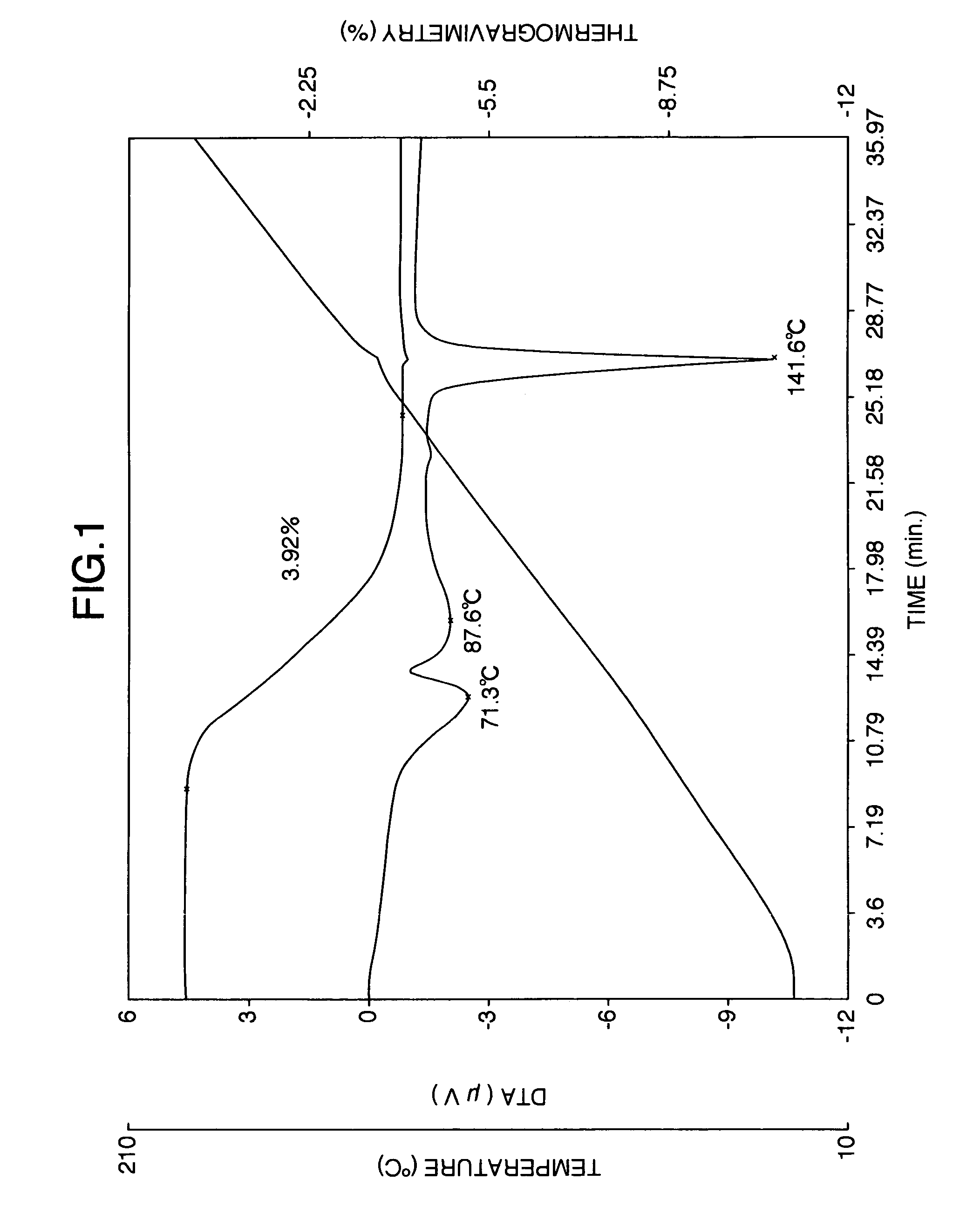
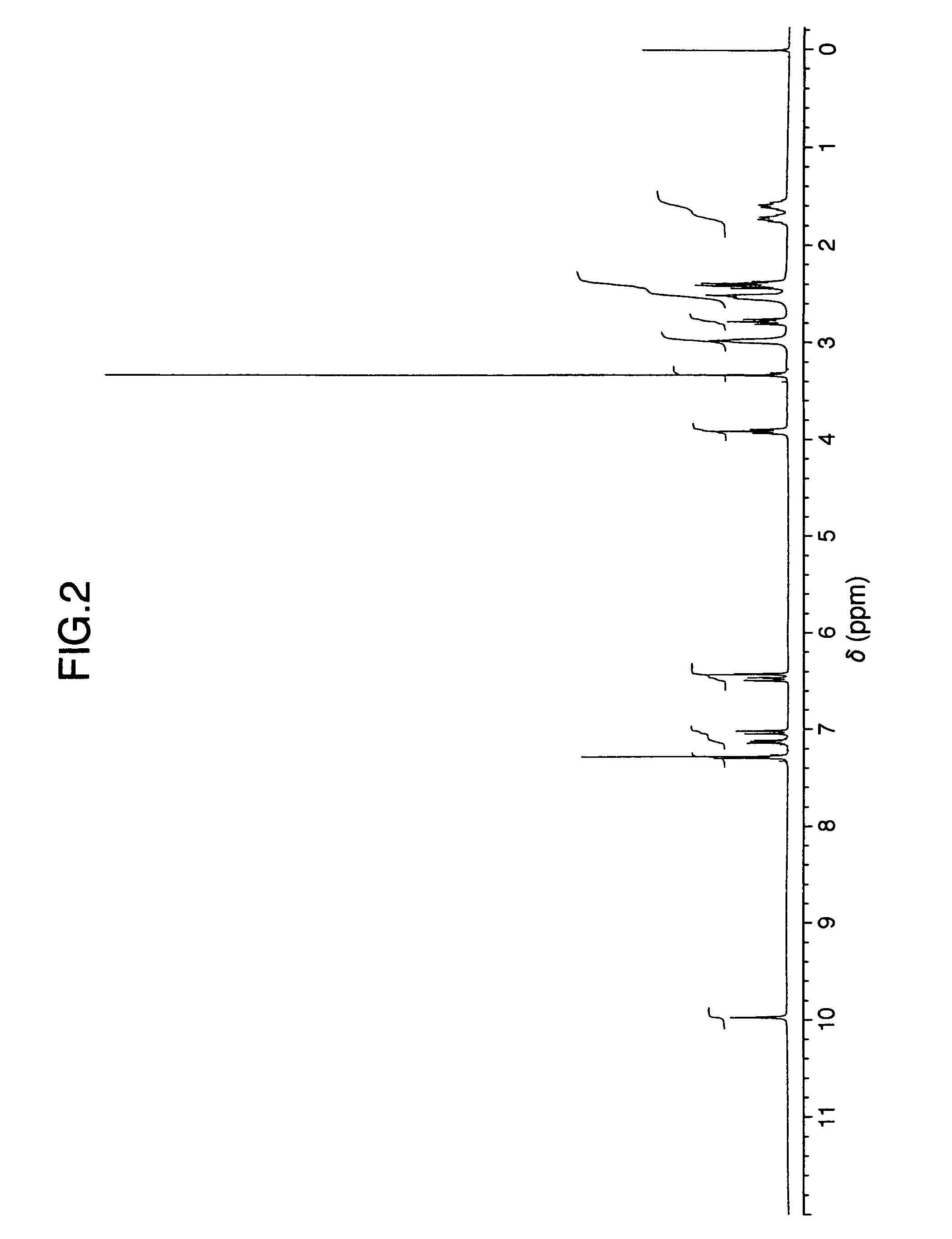
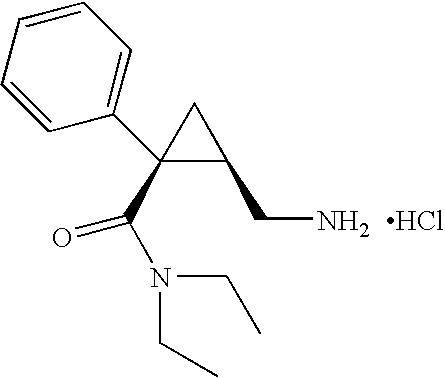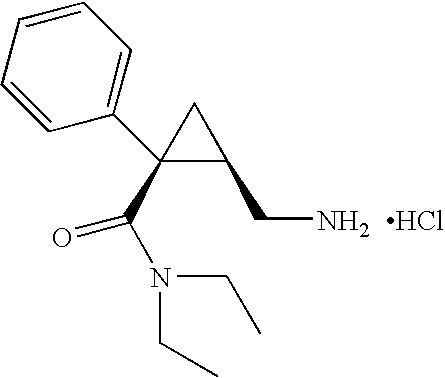Patents
Literature
47 results about "Milnacipran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Milnacipran is used to treat pain caused by a condition called fibromyalgia that affects the muscles, tendons, ligaments, and supporting tissues.
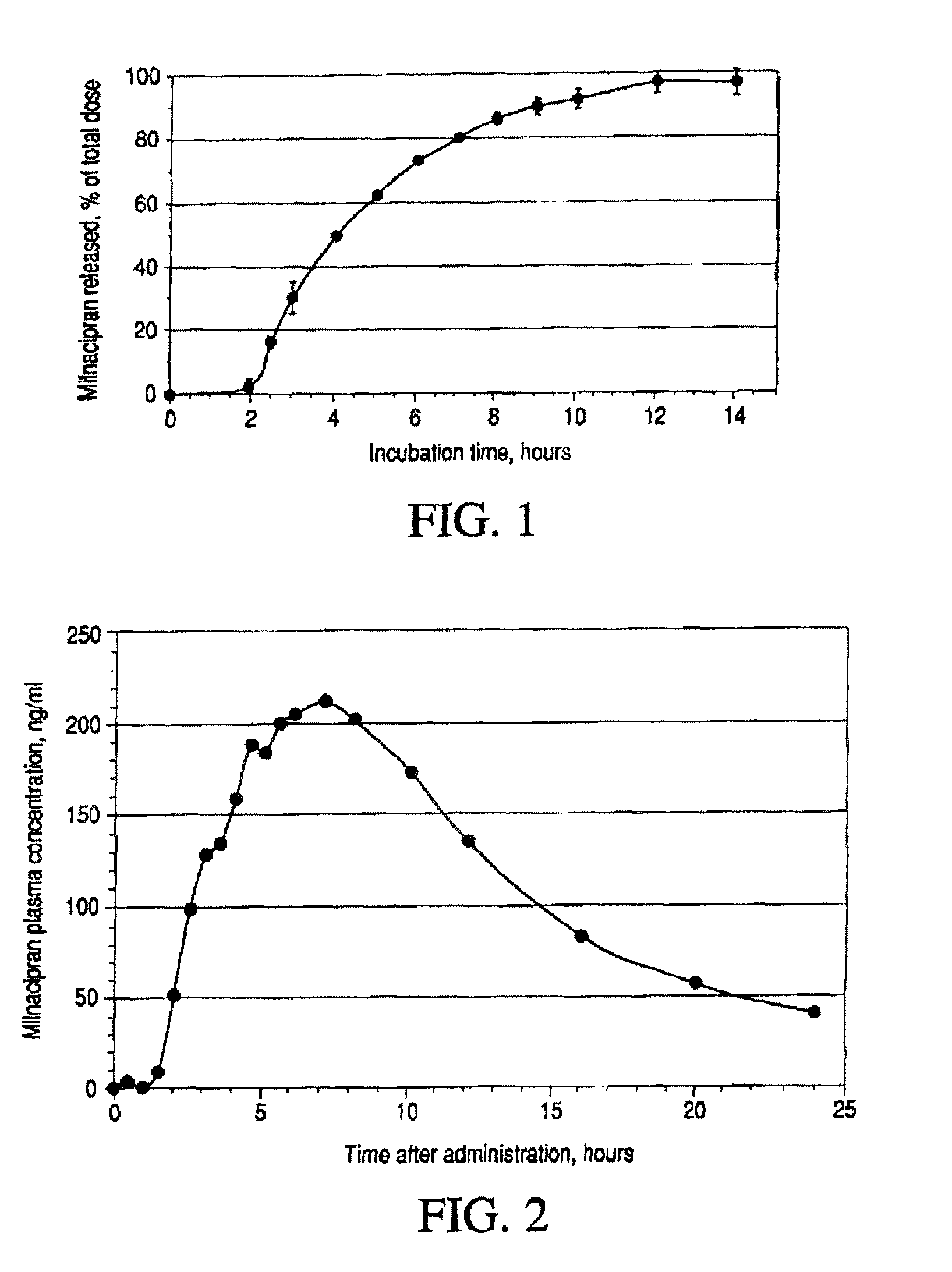
Modified release compositions of milnacipran
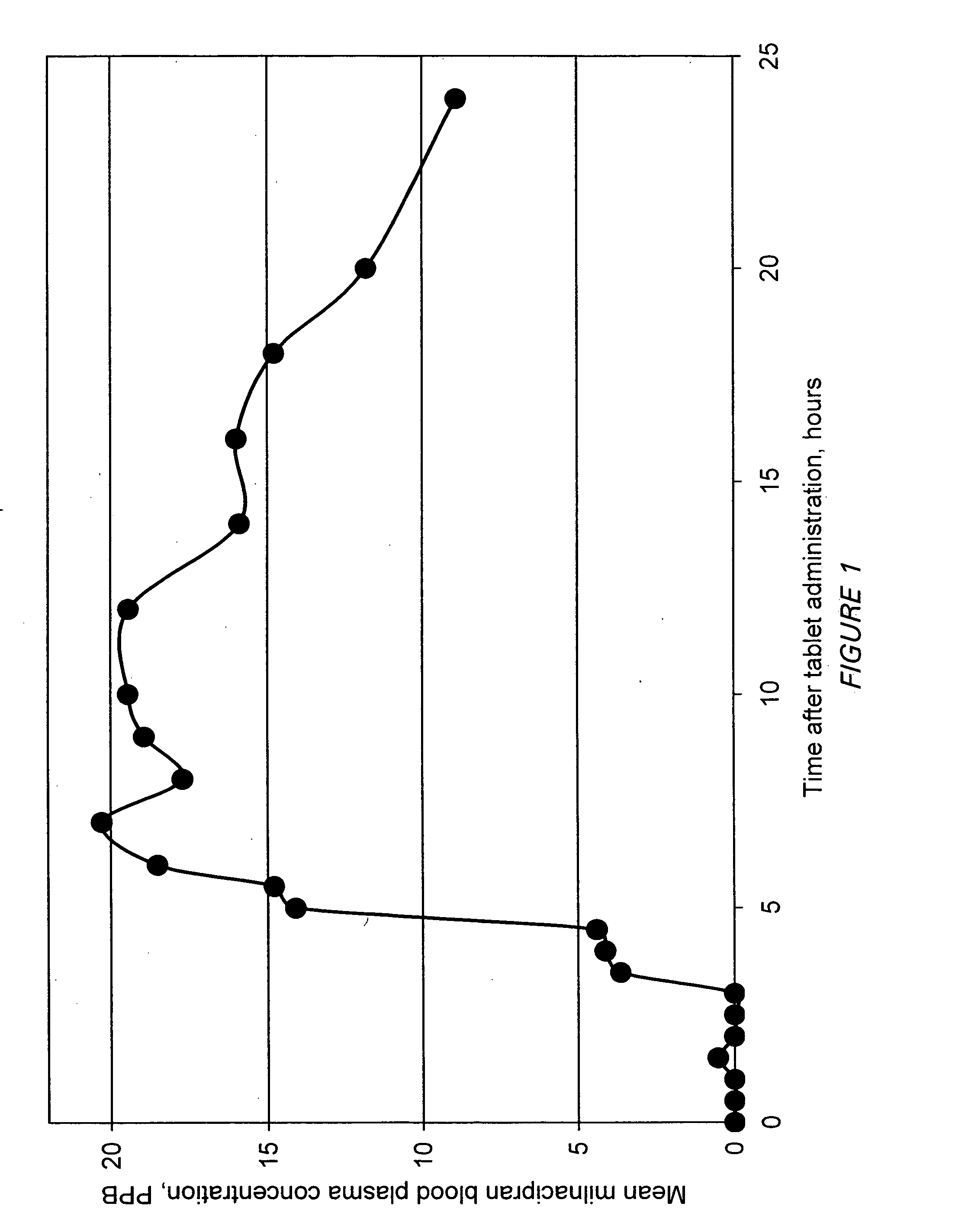
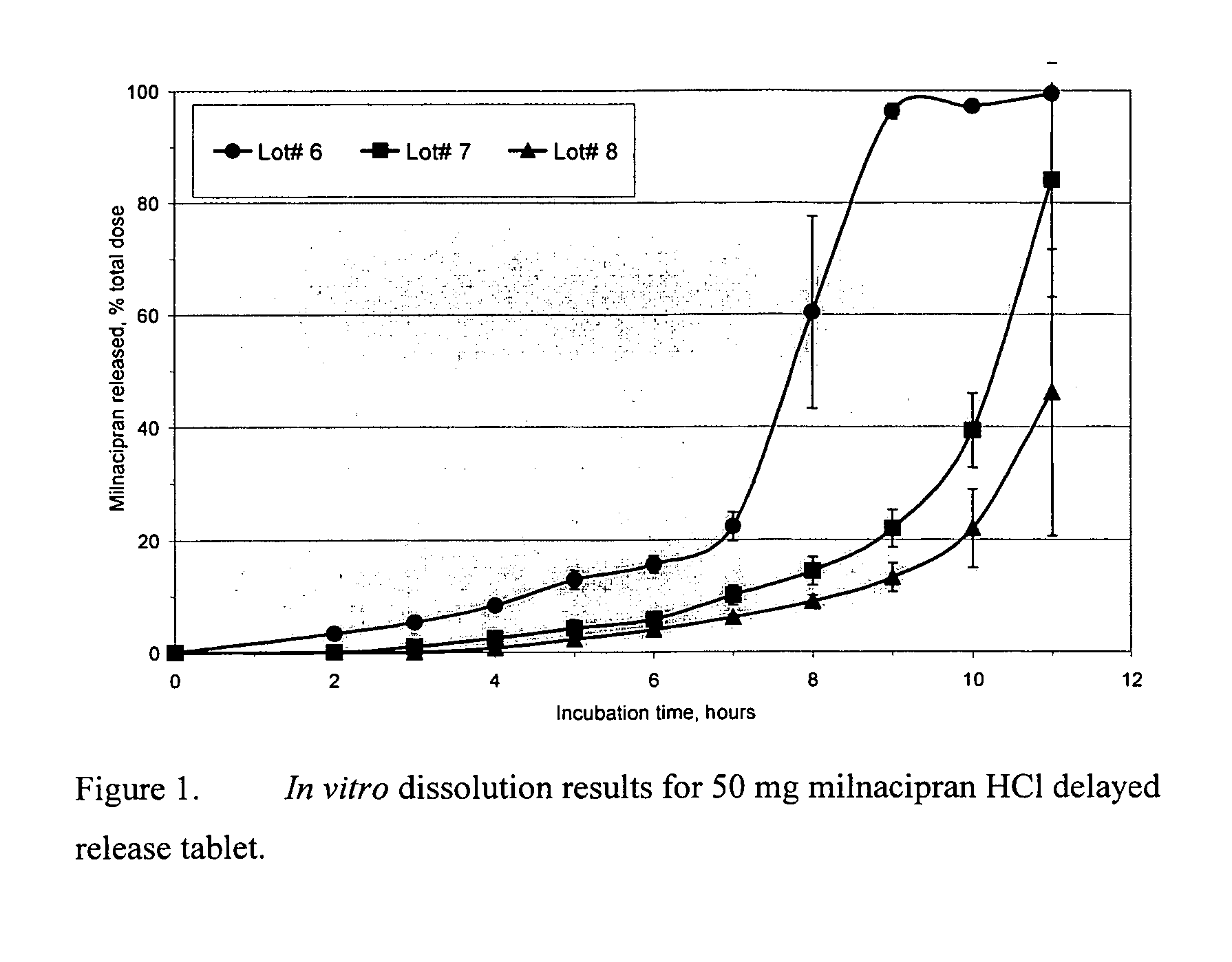
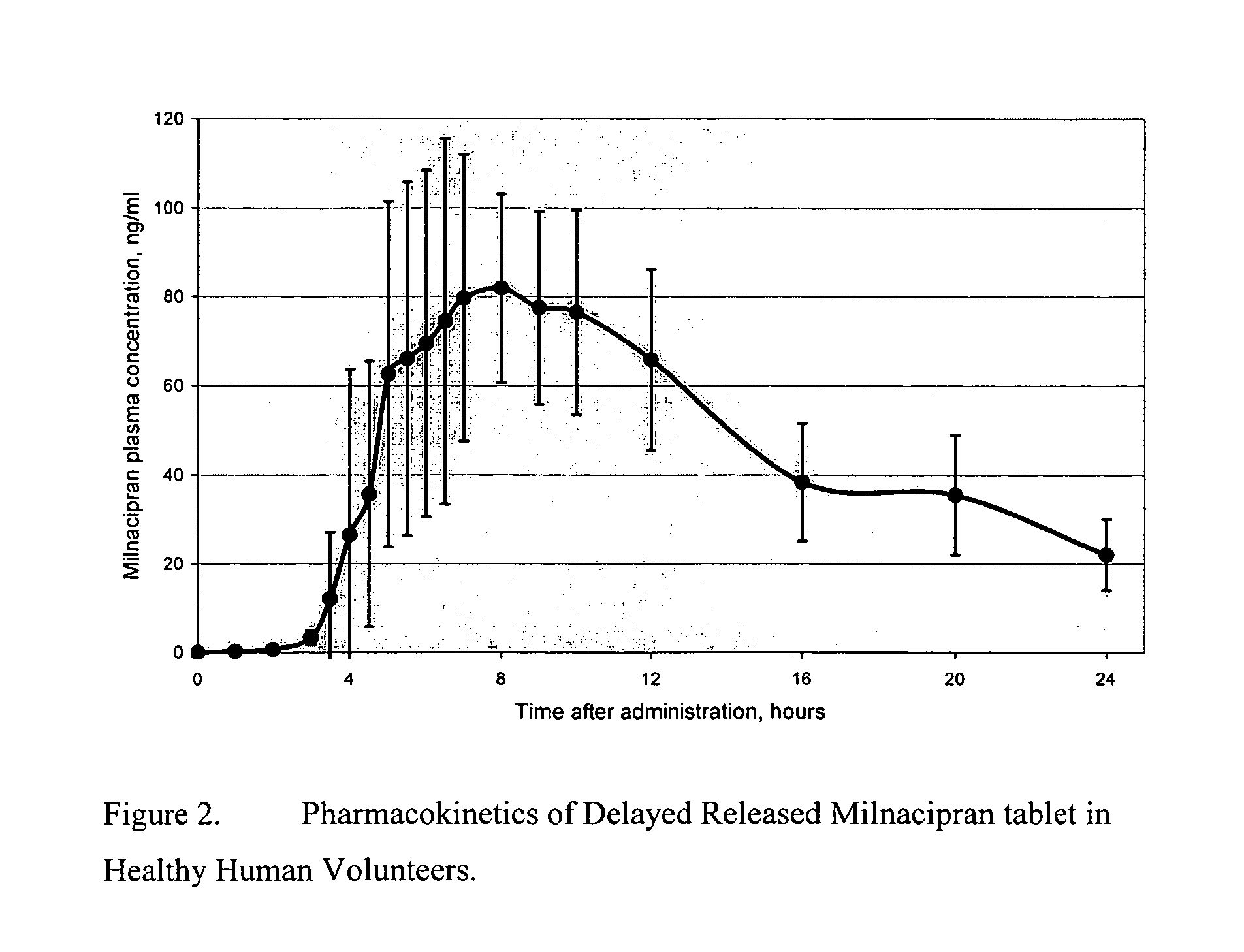
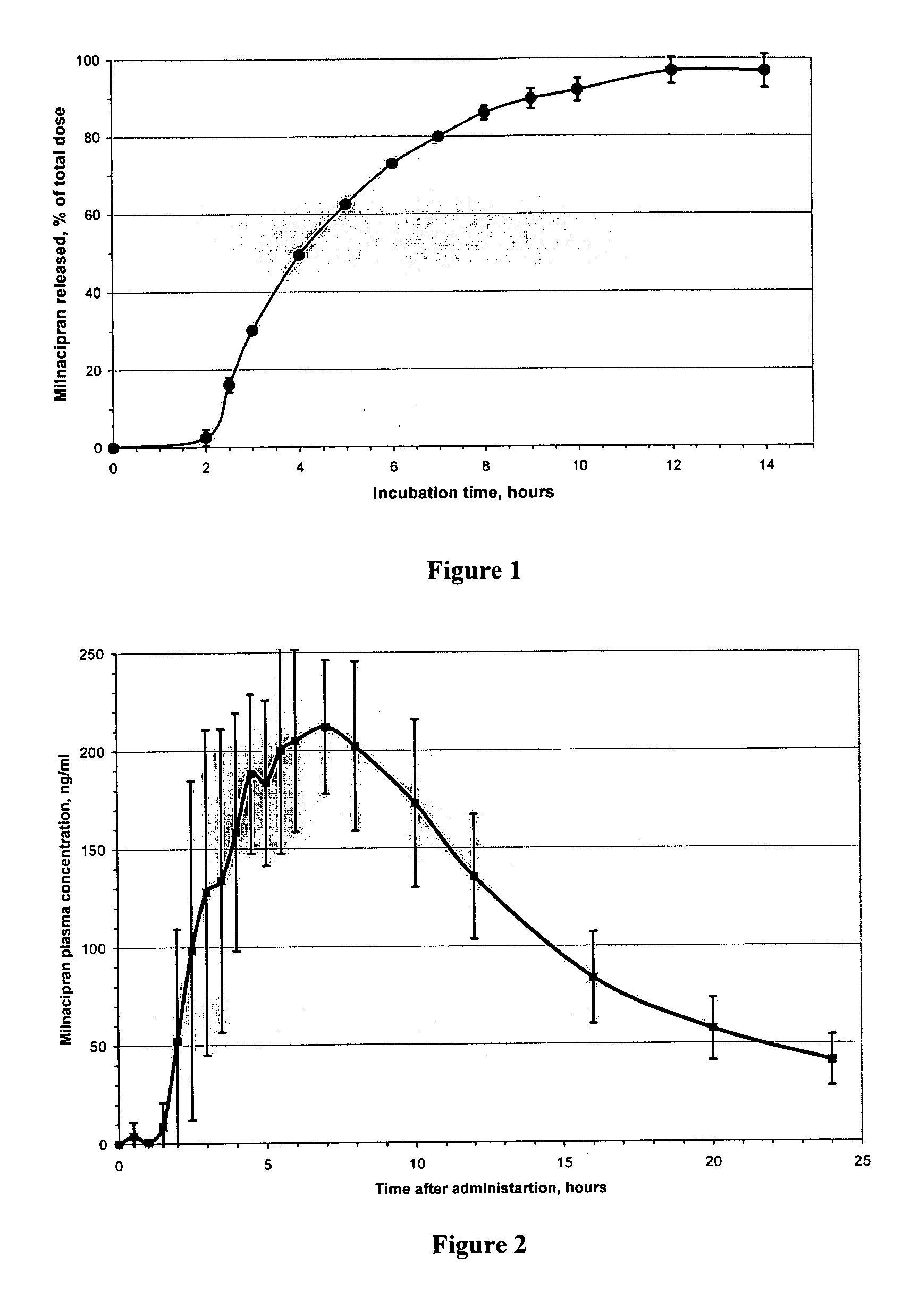
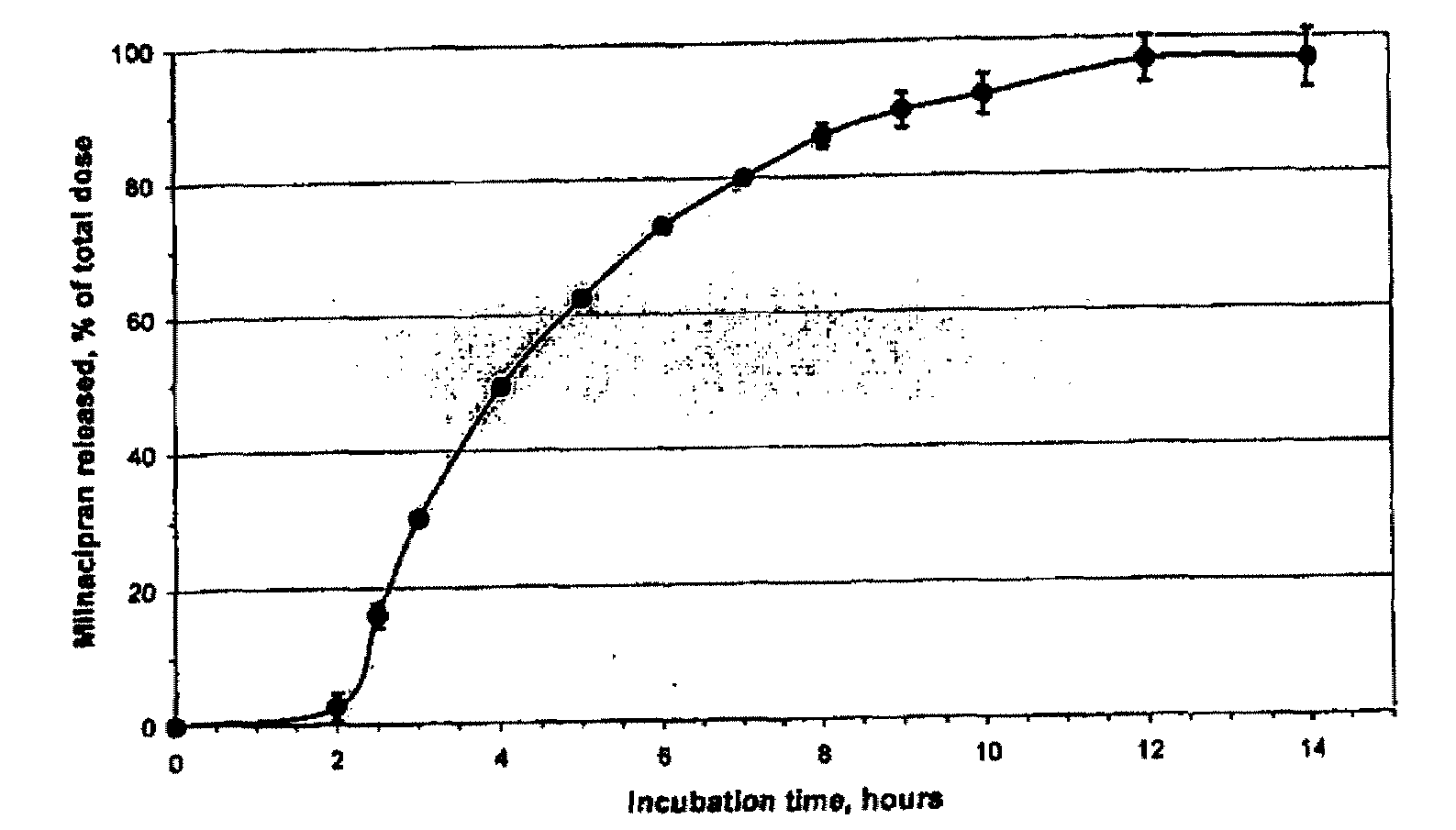
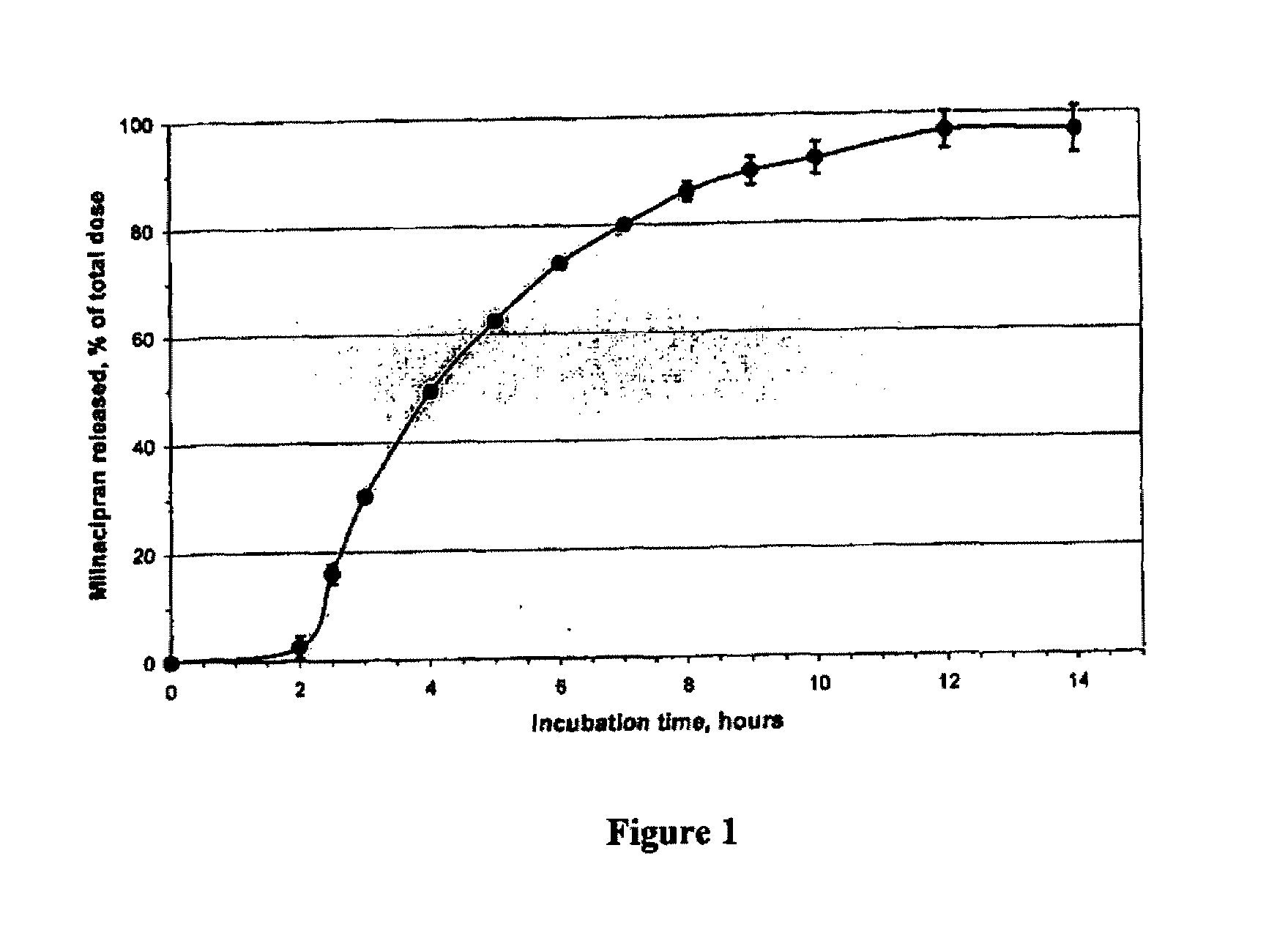
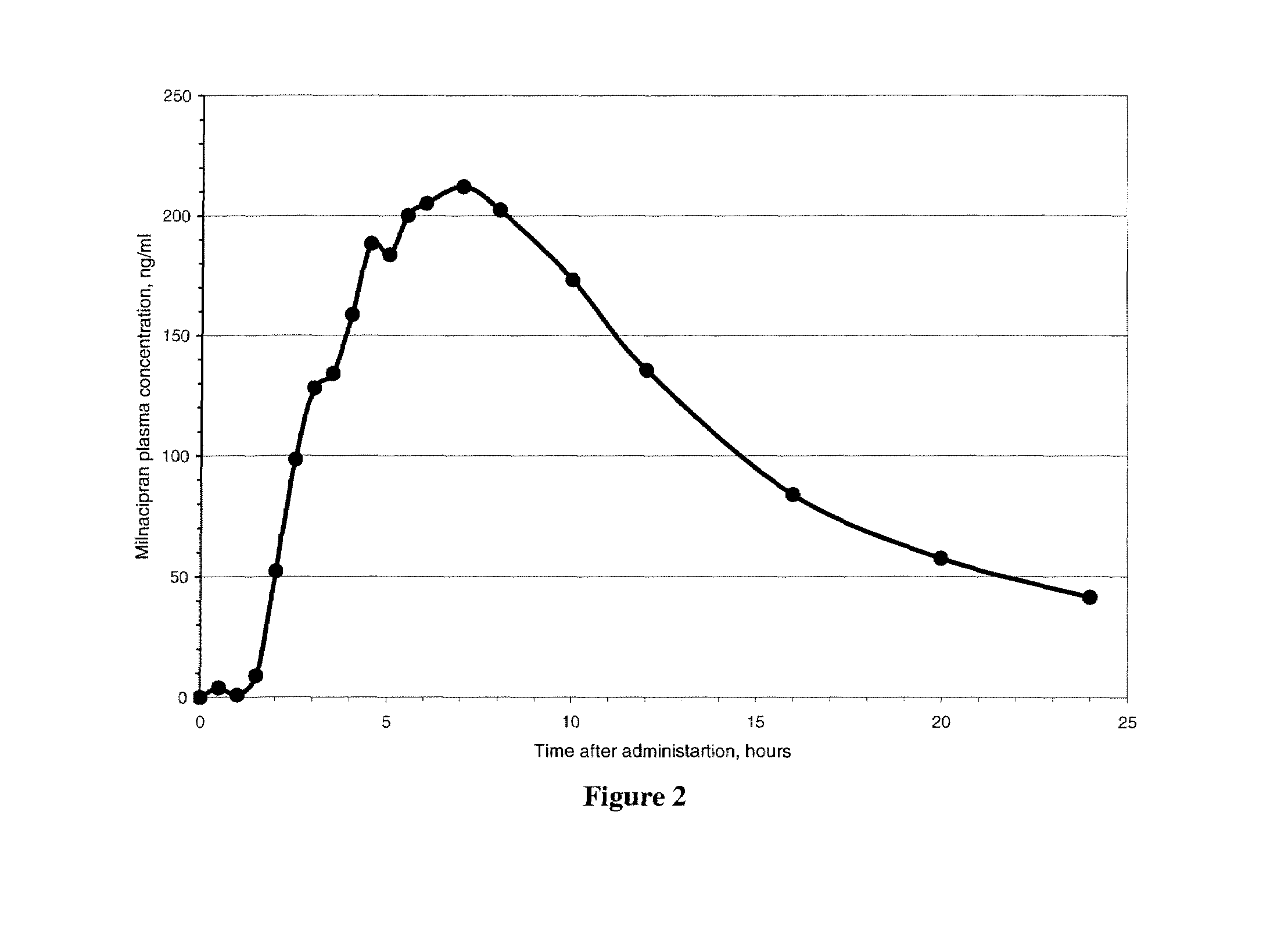
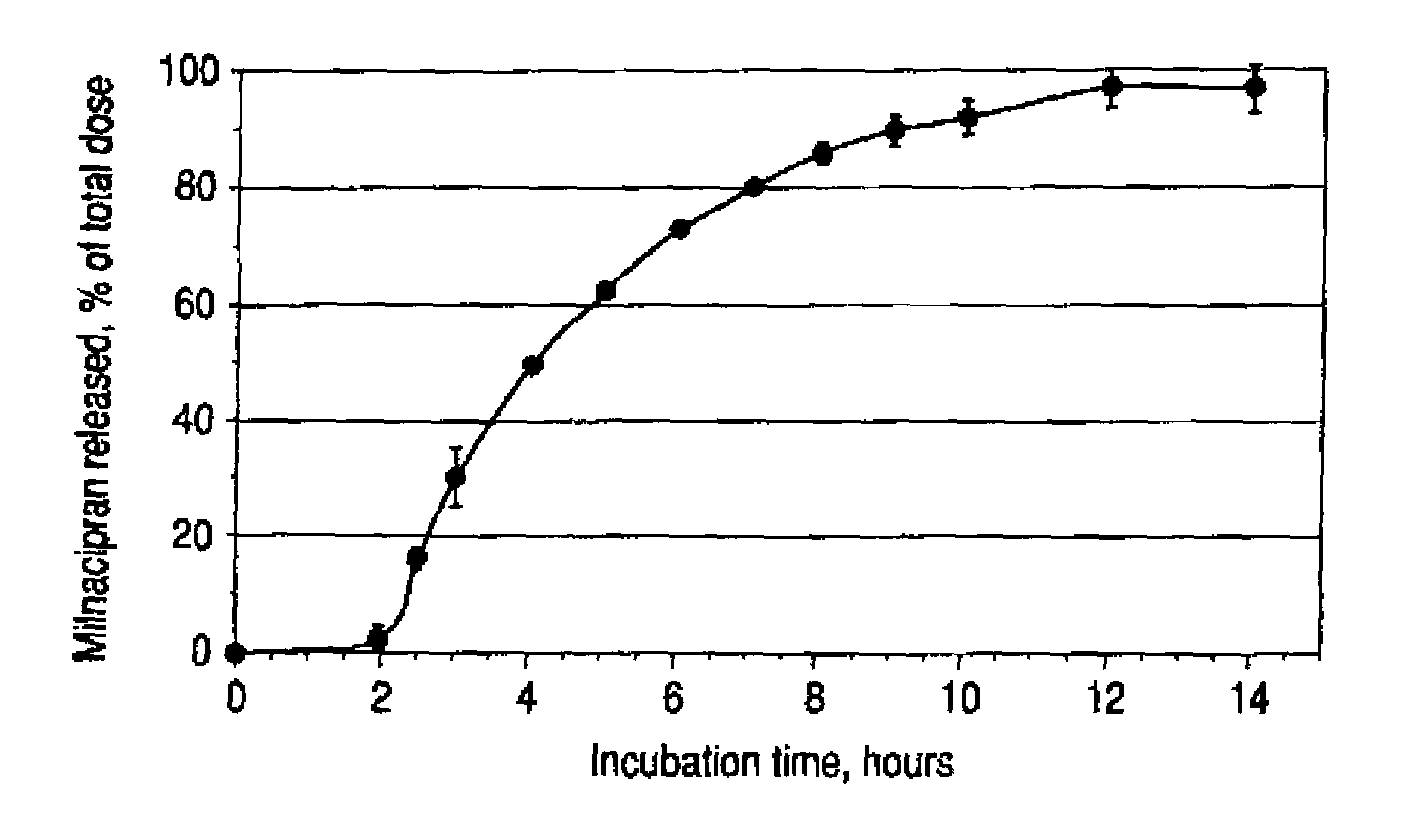
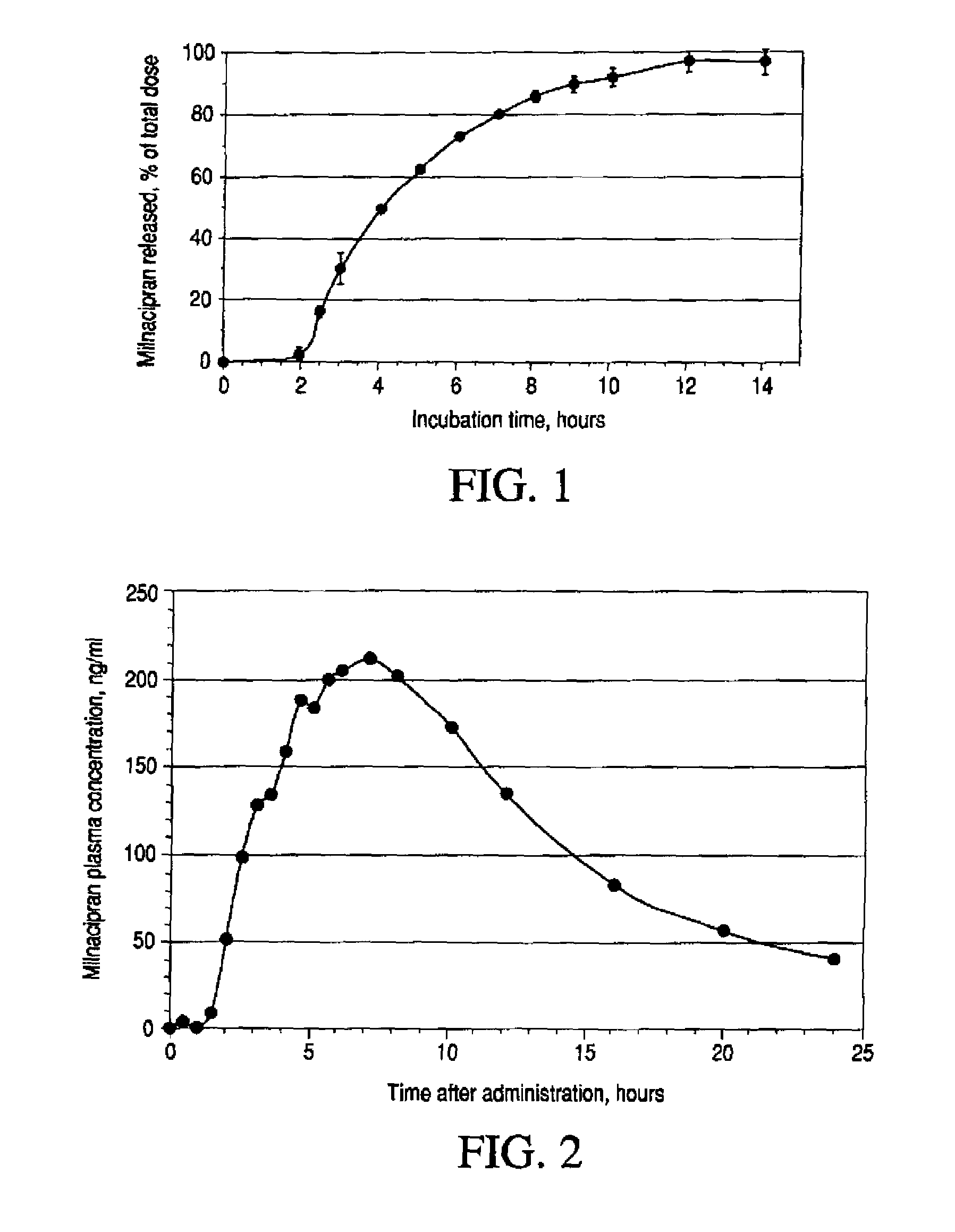
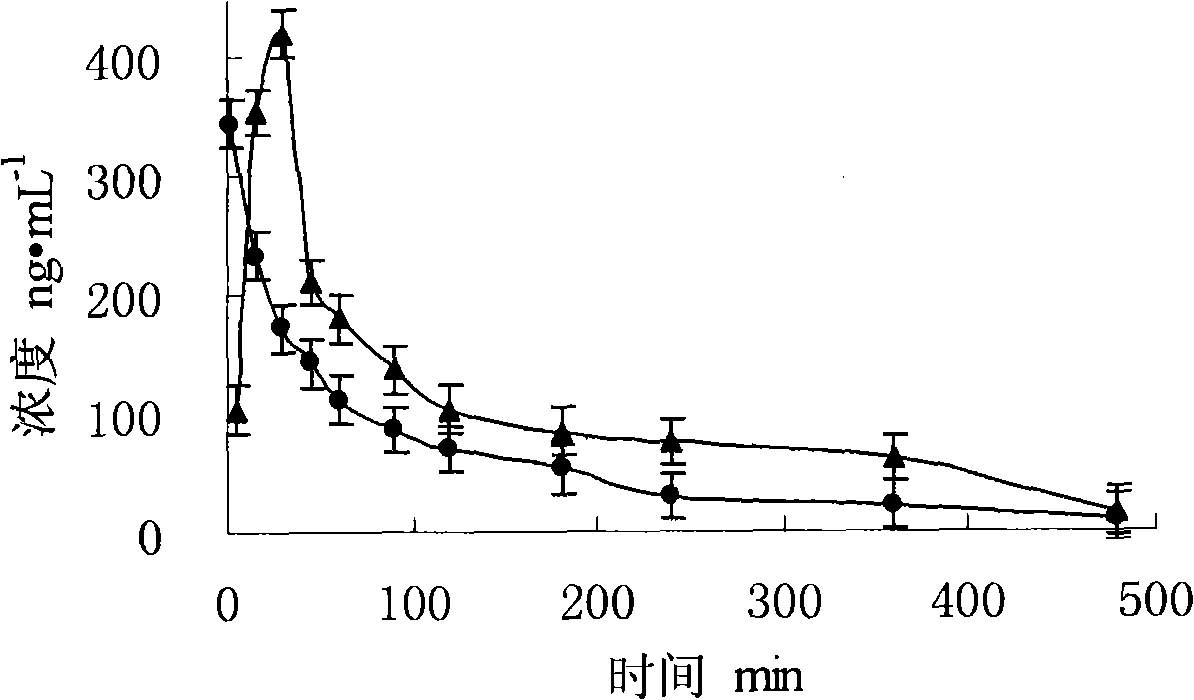
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Method of treating chronic fatigue syndrome
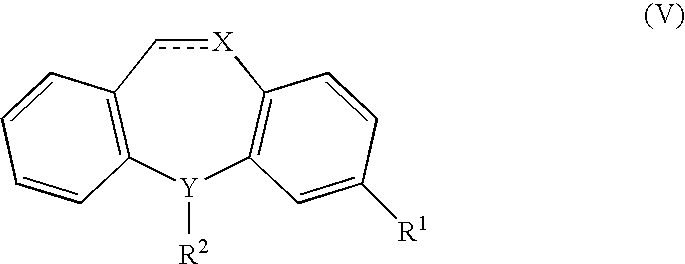
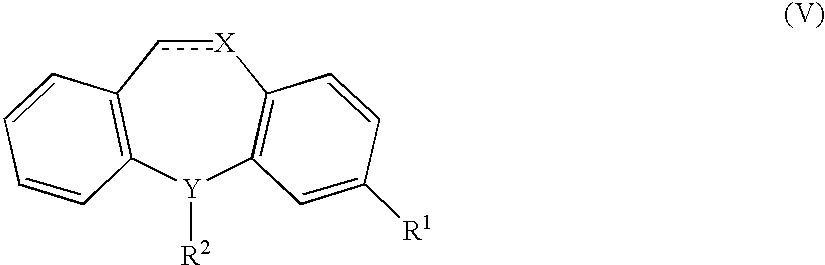
The present invention provides a method of treating fibromyalgia syndrome (FMS), chronic fatigue syndrome (CFS), and pain in an animal subject comprising administering a therapeutically effective amount of a dual serotonin norepinephrine reuptake inhibitor compound or a pharmaceutically acceptable salt thereof, wherein said dual serotonin norepinephrine reuptake inhibitor compound is characterized by a non-tricyclic structure and a greater inhibition of norepinephrine reuptake than serotonin reuptake, and wherein said compound is not administered adjunctively with phenylalanine or tyrosine. In particular, the use of milnacipran to treat FMS, CFS, and pain is disclosed.
Owner:FOREST LAB HLDG LTD
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Methods of treating fibromyalgia syndrome, chronic fatigue syndrome and pain
Owner:FOREST LAB HLDG LTD
Treatment of chronic pain associated with drug or radiation therapy
Methods for treating chronic widespread pain associated with drug therapy or radiation therapy are described. The method generally involves administering a therapeutically effective amount of a dual or tri reuptake inhibitor of a specific type or a pharmaceutically acceptable salt thereof. Preferably the compound is a non-tricyclic dual reuptake inhibitor. The most preferred compound is milnacipran or a bioequivalent or pharmaceutically acceptable salt thereof. Other preferred compounds are duloxetine and venlafaxine or a bioequivalent or pharmaceutically acceptable salt thereof. In yet another embodiment, a therapeutically effective amount of a non-tricyclic triple reuptake inhibitor (“TRI”) compound of a specific type, or a pharmaceutically acceptable salt thereof, is administered. The TRI compounds are characterized by their ability to block the reuptake (and, hence, increase central concentrations of) the three primary brain monoamines: serotonin, noradrenaline, and dopamine.
Owner:CYPRESS BIOSCI
Modified release compositions of milnacipran
InactiveUS20060024366A1Reduce incidenceDiminished decreased intensityCoatingsDrageesPalpitationsRash
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Methods of treating attention deficit/hyperactivity disorder (adhd)
The present invention provides a method of treating attention deficit / hyperactivity disorder (AD / HD) and associated tic disorders in an animal subject comprising administering an effective amount of an anti-AD / HD compound or a pharmaceutically acceptable salt thereof. The anti-AD / HD compound useful in the present invention is characterized by ant-AD / HD and anti-tic properties and exhibits at least two distinct pharmacological activities. In particular, the use of milnacipran to treat AD / HD and comorbid tic and psychiatric disorders is disclosed.
Owner:CYPRESS BIOSCI
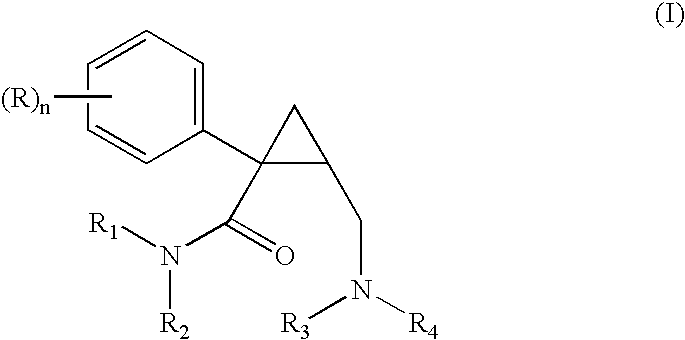
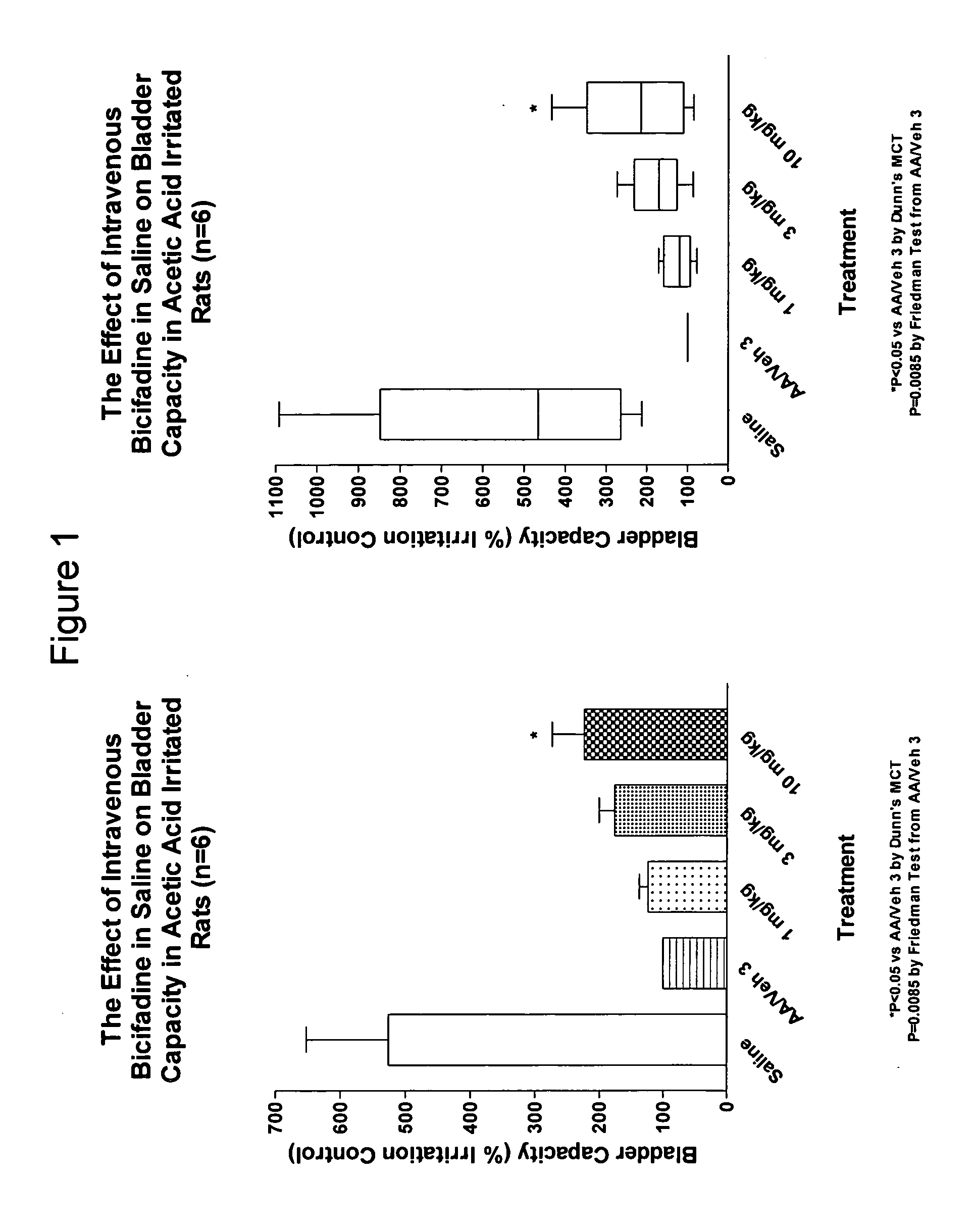
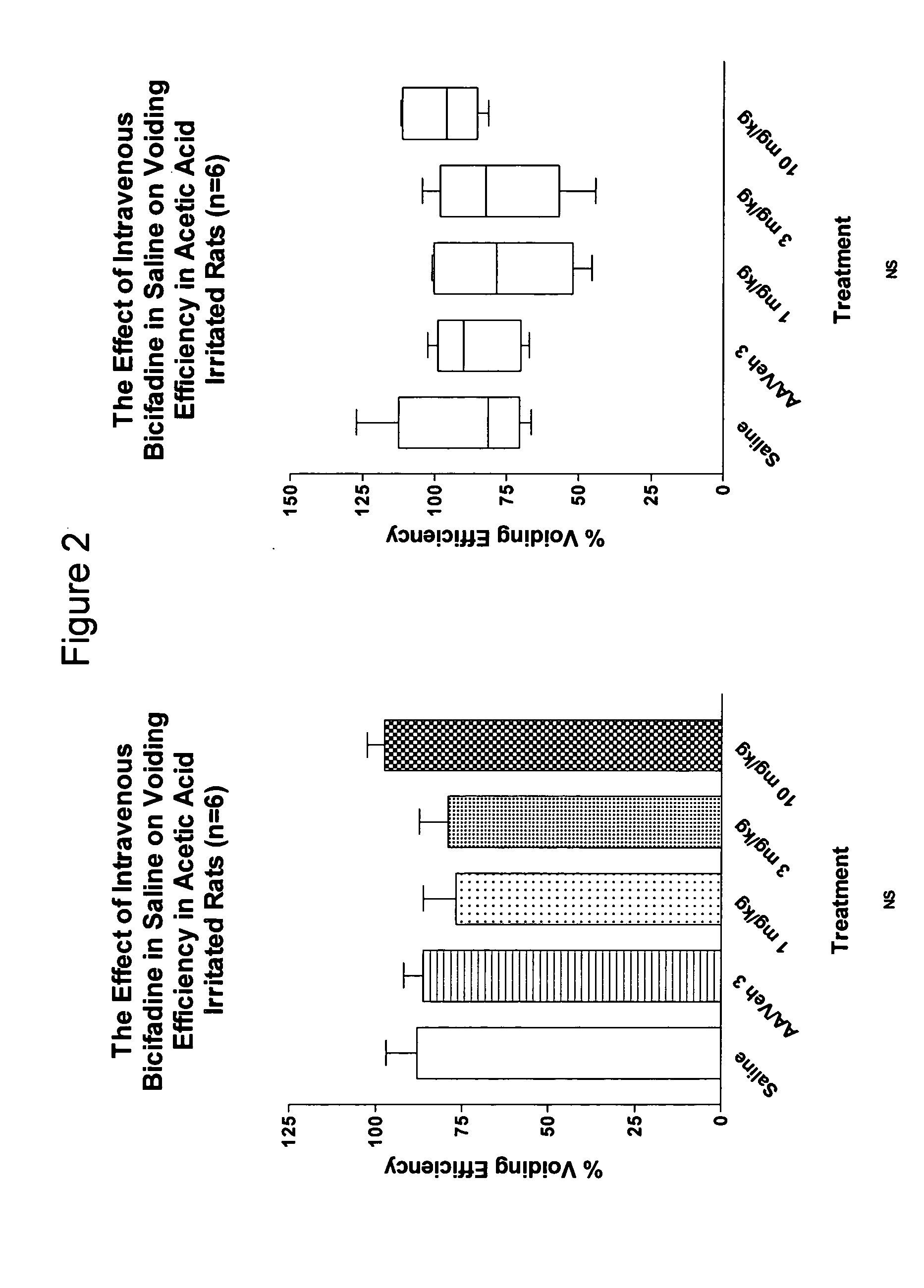
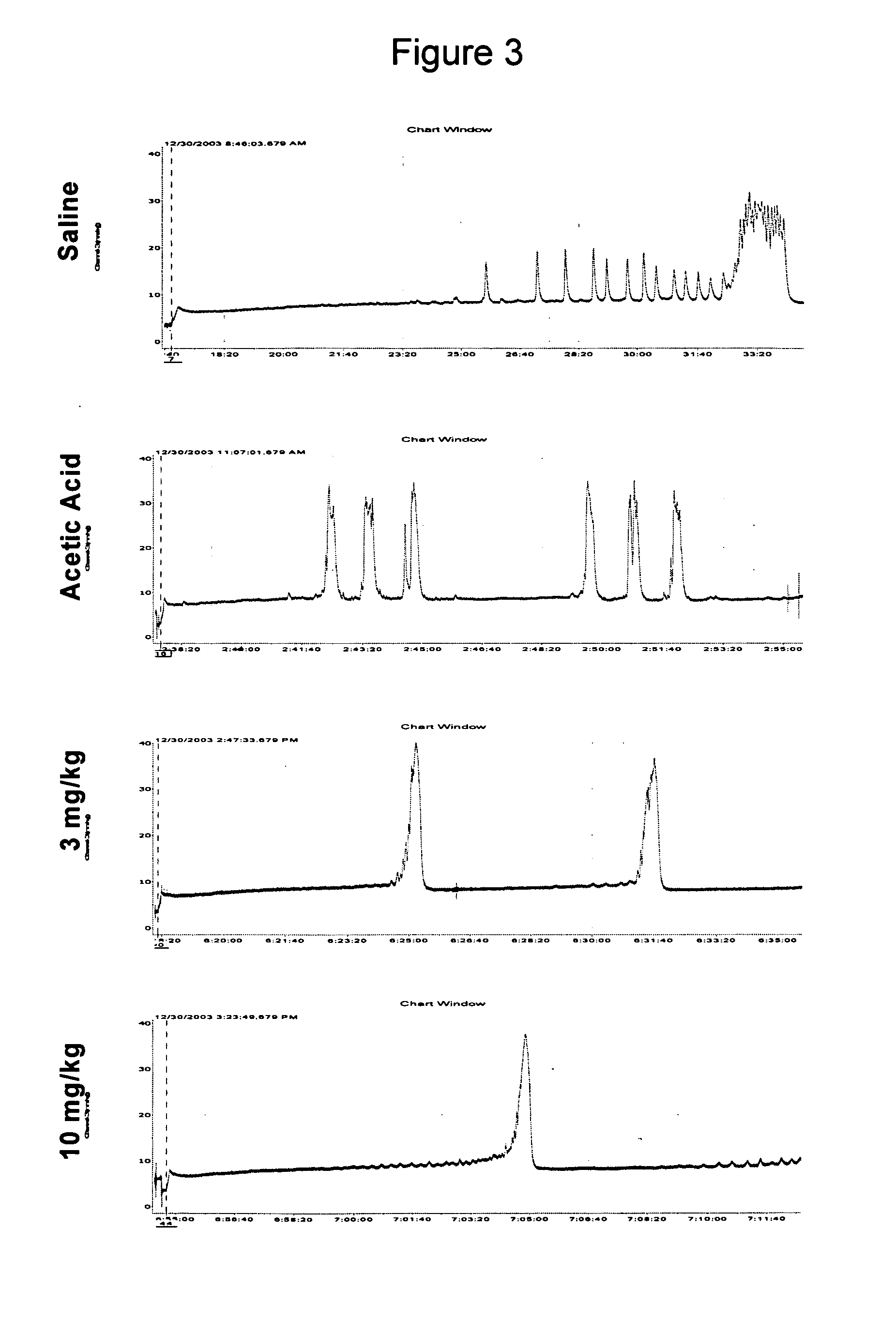
Dual acting SNRI-NMDA antagonists for the treatment of genitourinary disorders
InactiveUS20050282859A1Improve efficiencyBenefit of the therapyBiocideAnimal repellantsBicifadineDisease
Discolsed herein are compositions and methods for treatment of genitourinary disorders (e.g., urge incontinence). The compositions may generally include a dual-acting SNRI-NMDA antagonist (e.g., bicifadine and / or milnacipran). Alternatively, the compositions may generally include an SNRI and an NMDA antagonist.
Owner:EDUSA PHARMA
Prevention and treatment of functional somatic disorders, including stress-related disorders
InactiveUS20090105222A1Improve concentrationBiocideNervous disorderNorepinephrine reuptake inhibitorStress-related disorders
Methods for the prevention or treatment of stress-related disorders by administering a therapeutically effective amount of a dual serotonin / norepinephrine reuptake inhibitor to an individual under stress are described. A triple monoamine reuptake inhibitor for serotonin / noradrenaline / dopamine may also be administered to an individual at risk for a stress-related disorder. In a preferred embodiment the compound is milnacipran and is prophylactically administered at an effective amount to delay or prevent stress-related disorders in an individual at risk.
Owner:CYPRESS BIOSCI
Modified release compositions of milnacipran
InactiveUS20090018203A1Reduce incidenceDiminished decreased intensityOrganic active ingredientsBiocideExcipientPharmaceutical preservatives
A milnacipran formulation that provides delayed and extended release of milnacipran has been developed. The formulation comprises milnacipran or a salt thereof; an extended release excipient, and a delayed release excipient. The formulation provides, upon administration to a human subject, a Tmax of 4-10 hours.
Owner:COLLEGIUM PHARMA INC
Milnacipran for the long-term treatment of fibromyalgia syndrome
ActiveUS20070072946A1Effective long-term treatmentEffective and long-term treatmentBiocideNervous disorderLong term treatmentsSerotonin reuptake
The present invention is directed to methods for providing long-term treatment of fibromyalgia syndrome (FMS) by administering a dual re-uptake inhibitor to a patient with FMS. More particularly, the present invention is directed to the long-term treatment of FMS by administering a norepinephrine-serotonin reuptake inhibitor (NSRI) to a patient with FMS.
Owner:FOREST LAB HLDG LTD
Milnacipran for the treatment of fatigue associated with fibromyalgia syndrome
InactiveUS20080058317A1Effective and long-term treatmentEffective and treatmentBiocideNervous disorderChronic Widespread PainLong term treatments
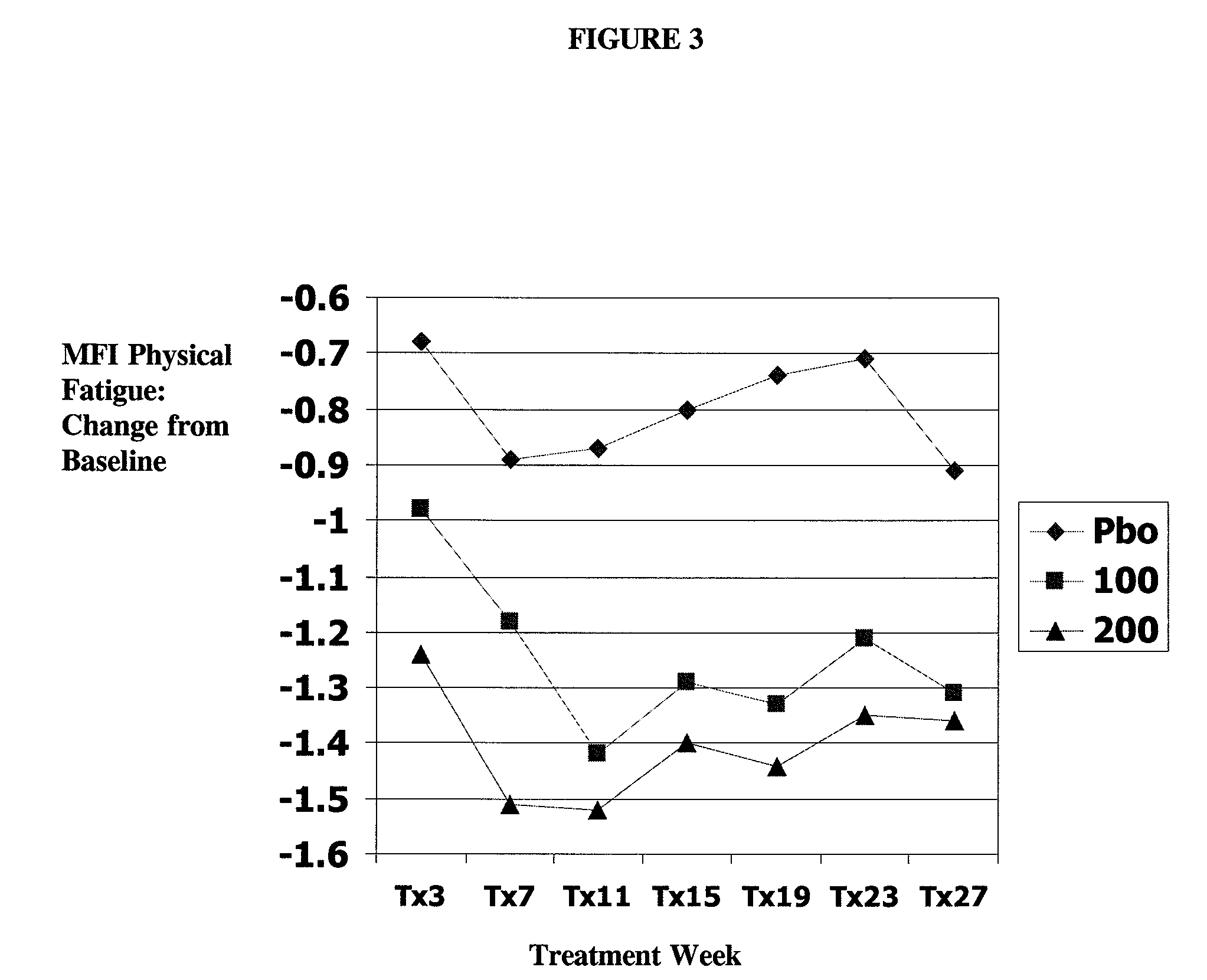
Methods for treating fatigue associated with fibromyalgia by administering high-dose milnacipran to a patient suffering from such fatigue are provided. Also provided are methods for the long-term treatment of fatigue associated with FMS by administering milnacipran to a patient suffering from such fatigue.
Owner:CYPRESS BIOSCI
Modified release compositions of milnacipran
InactiveUS7704527B2Diminished incidence and decreased intensityImprove sweatingBiocideOrganic active ingredientsExcipientMilnacipran
A milnacipran formulation that provides delayed and extended release of milnacipran has been developed. The formulation comprises milnacipran or a salt thereof; an extended release excipient, and a delayed release excipient. The formulation provides, upon administration to a human subject, a Tmax of 4-10 hours.
Owner:COLLEGIUM PHARMA INC
Methods of treating fibromyalgia syndrome, chronic fatigue syndrome and pain
The present invention provides a method of treating fibromyalgia syndrome (FMS), chronic fatigue syndrome (CFS), and pain in an animal subject. The method generally involves administering a therapeutically effective amount of a dual serotonin norepinephrine reuptake inhibitor compound or a pharmaceutically acceptable salt thereof, wherein said dual serotonin norepinephrine reuptake inhibitor compound is characterized by a non-tricyclic structure and an equal or greater inhibition of norepinephrine reuptake than serotonin reuptake. In particular, the use of milnacipran to treat FMS, CFS, and pain is disclosed.
Owner:FOREST LAB HLDG LTD
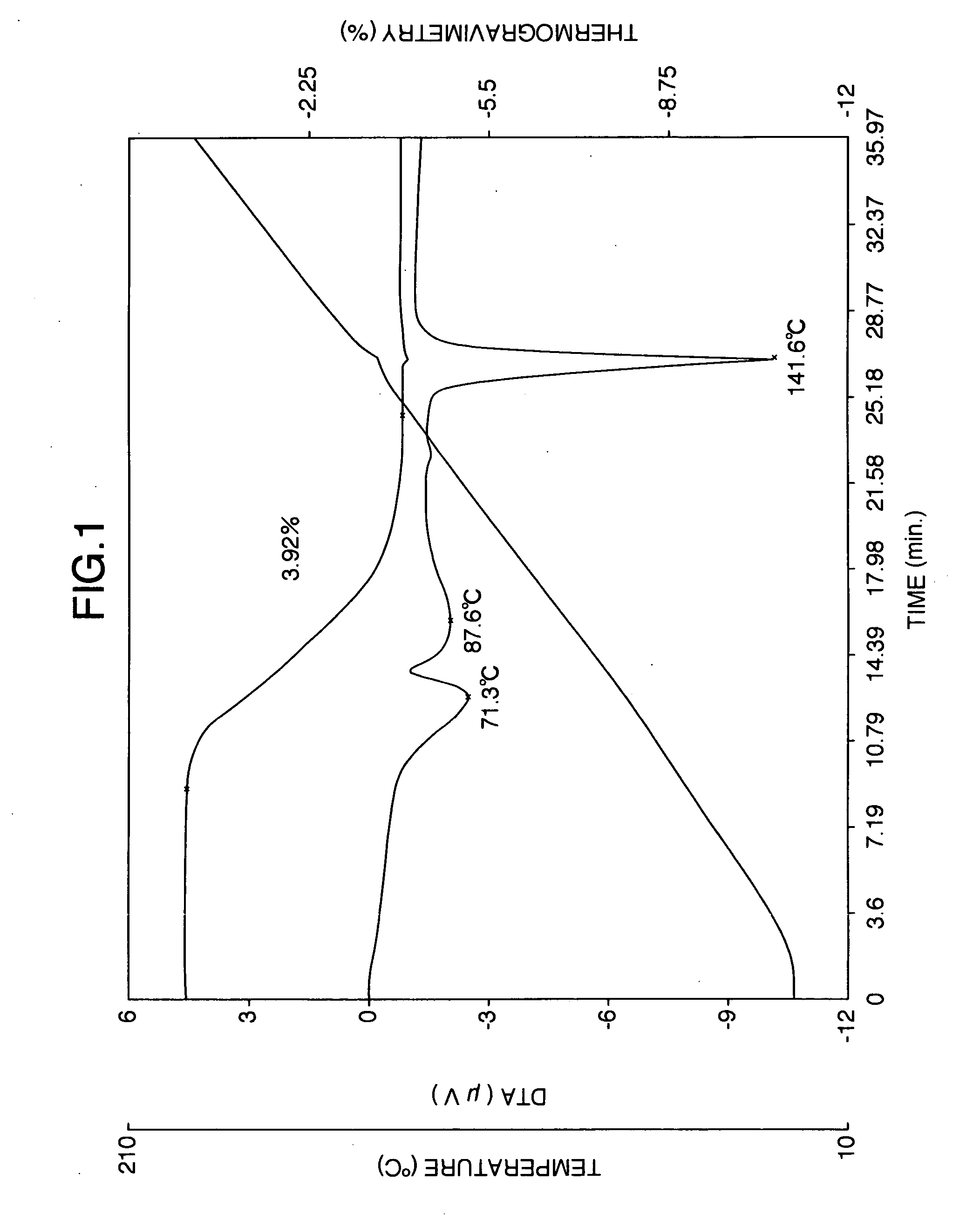
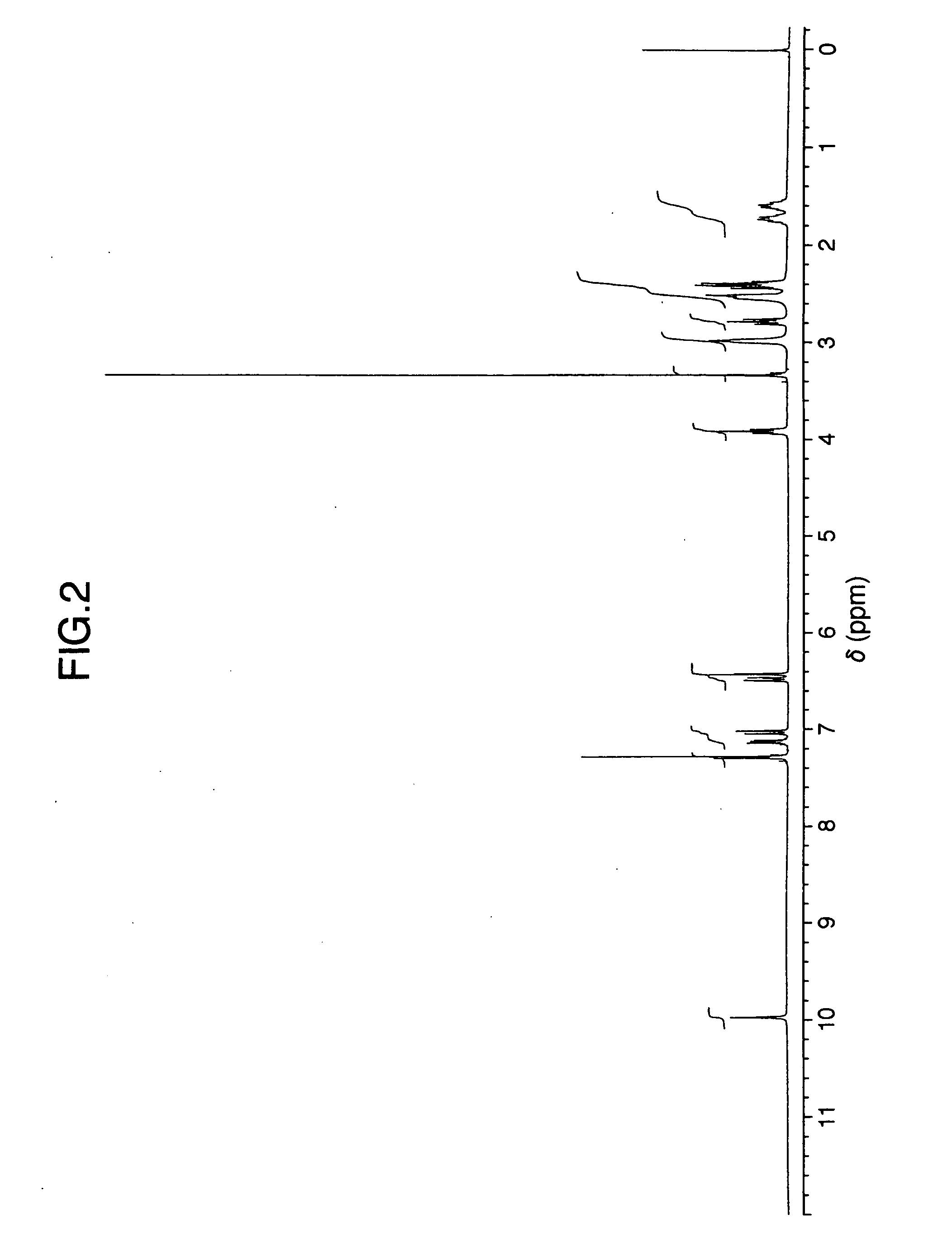
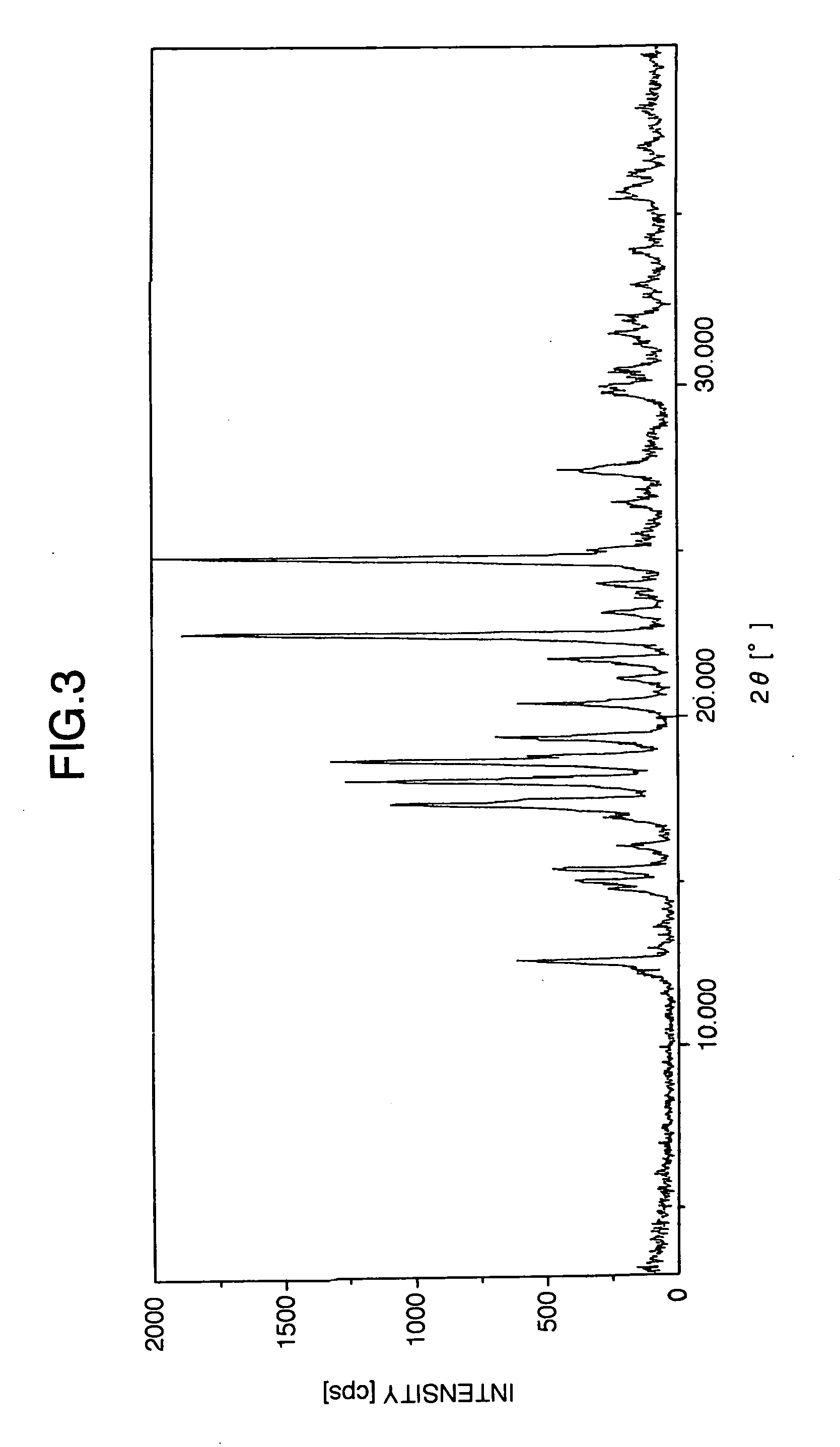
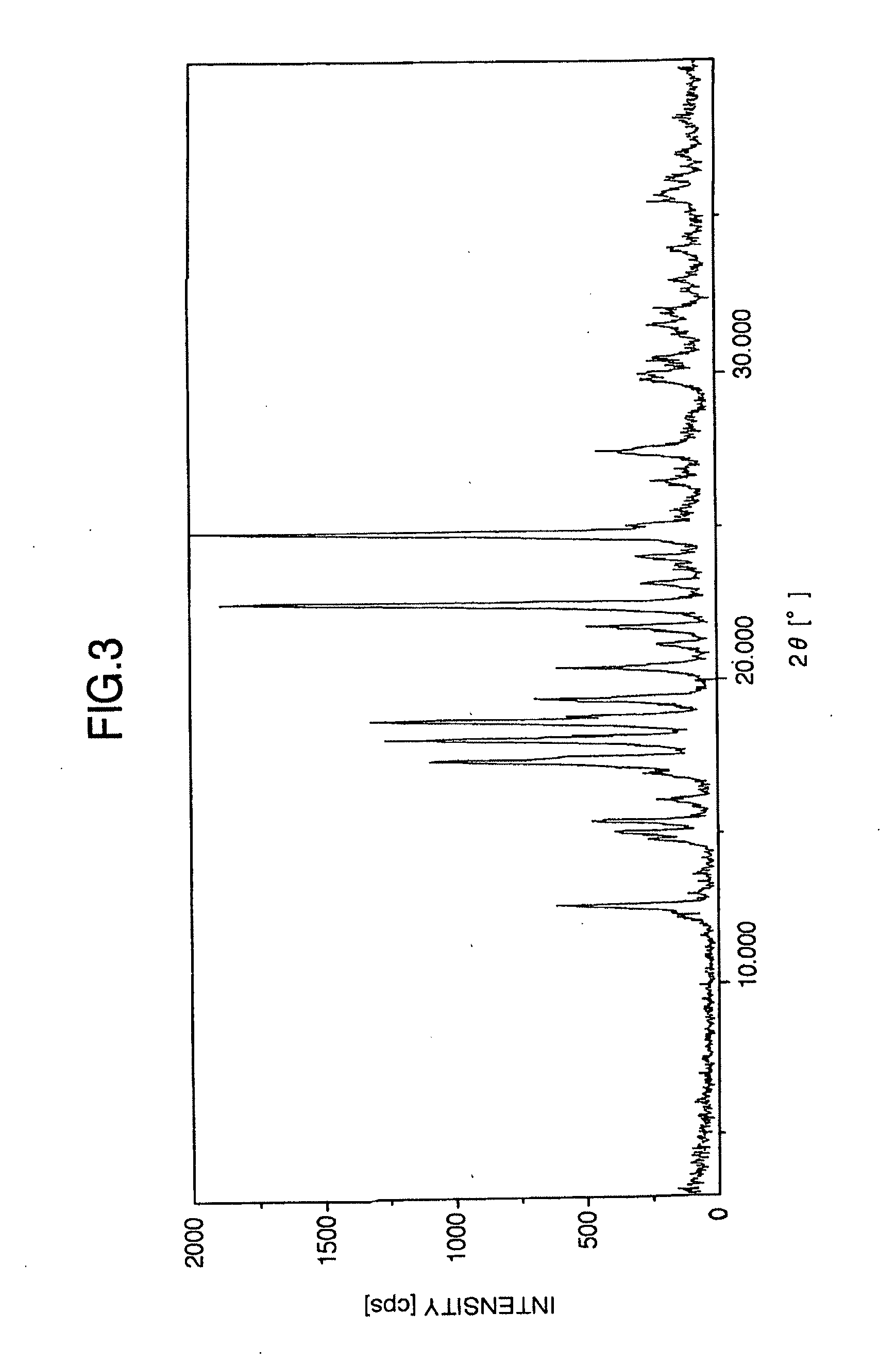
Stabilized milnacipran formulation
ActiveUS20090049935A1Prevent adhesionPrevent coagulationBiocideOrganic active ingredientsMilnacipranChemistry
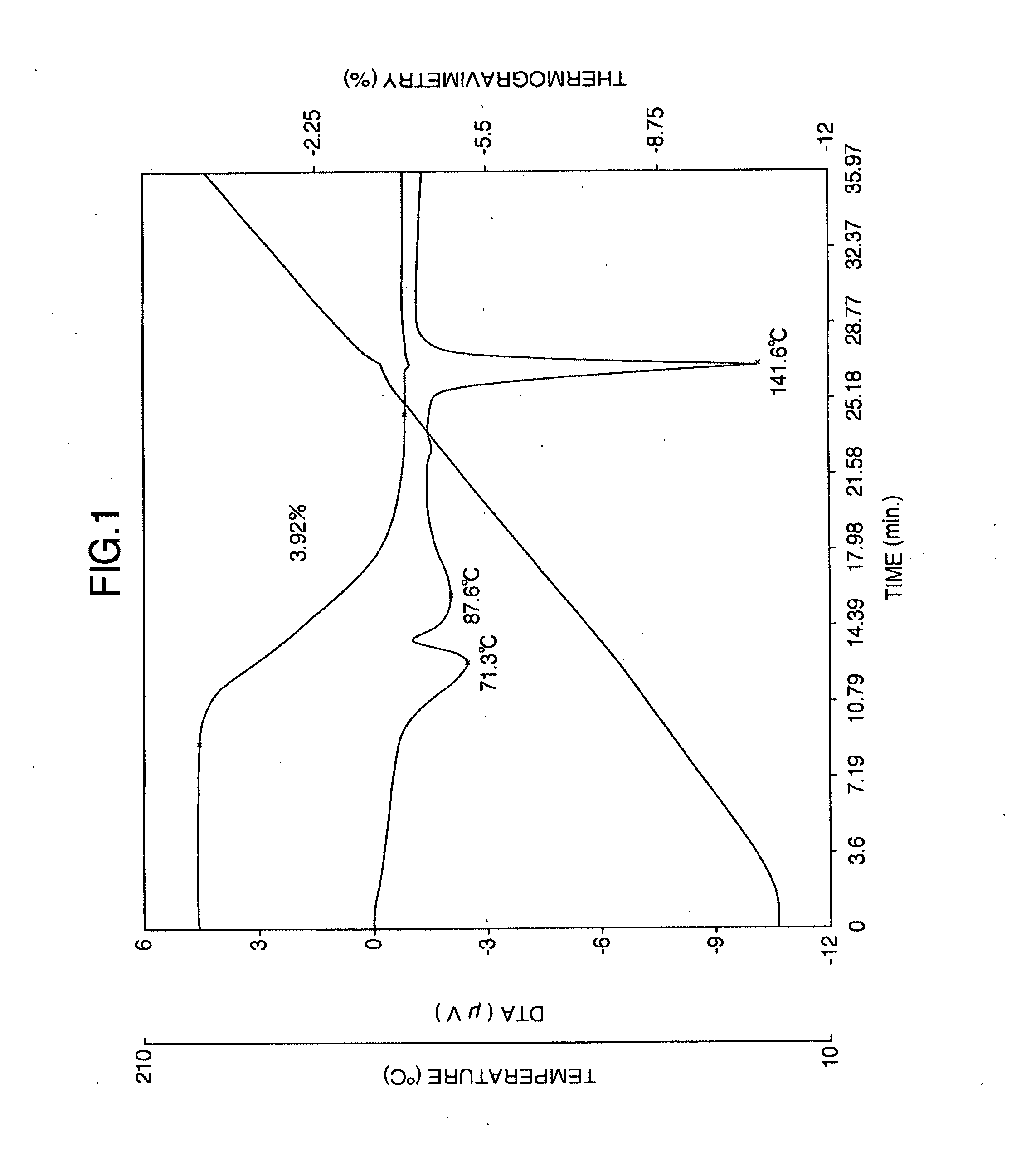
It is intended to provide a milnacipran formulation which is more stable than a conventionally known milnacipran formulation, and a method of stabilizing a milnacipran formulation. The object could be achieved by using a milnacipran-containing composition in which milnacipran or a salt thereof is allowed to exist in a porous carrier, packing a powder containing milnacipran or a salt thereof in an HPMC capsule, or combining an additive which does not cause an interaction with milnacipran with time.
Owner:PIERRE FABRE MEDICAMENT SAS
Method of treating chronic fatigue syndrome
InactiveUS20030139476A1Achieve benefitsEffective amount for useBiocideNervous disorderDepressantTyrosine
The present invention provides a method of treating fibromyalgia syndrome (FMS), chronic fatigue syndrome (CFS), and pain in an animal subject comprising administering a therapeutically effective amount of a dual serotonin norepinephrine reuptake inhibitor compound or a pharmaceutically acceptable salt thereof, wherein said dual serotonin norepinephrine reuptake inhibitor compound is characterized by a non-tricyclic structure and a greater inhibition of norepinephrine reuptake than serotonin reuptake, and wherein said compound is not administered adjunctively with phenylalanine or tyrosine. In particular, the use of milnacipran to treat FMS, CFS, and pain is disclosed.
Owner:FOREST LAB HLDG LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
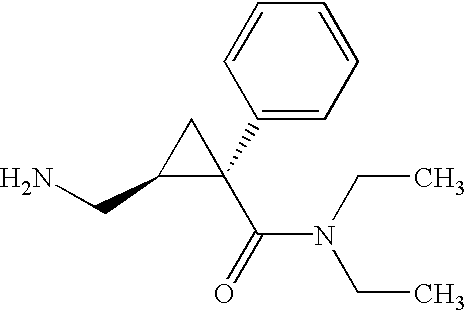
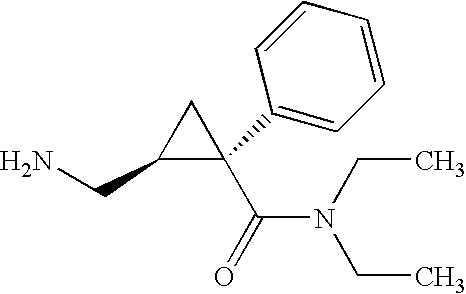
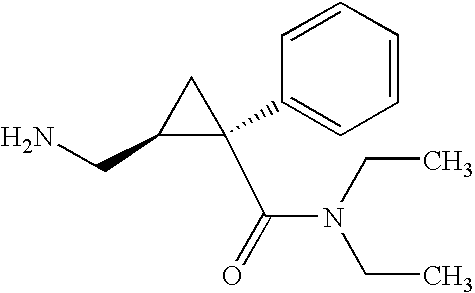
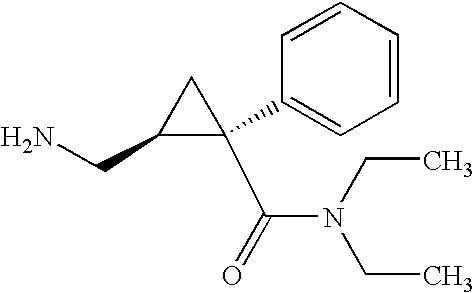
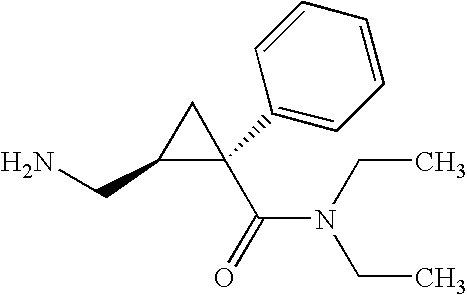
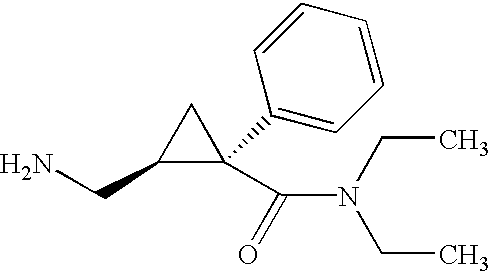
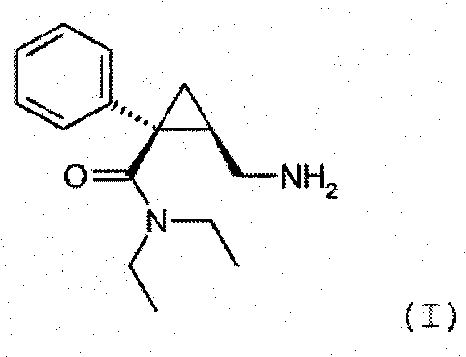
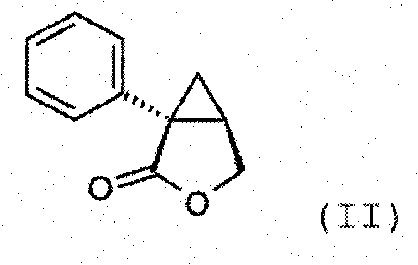
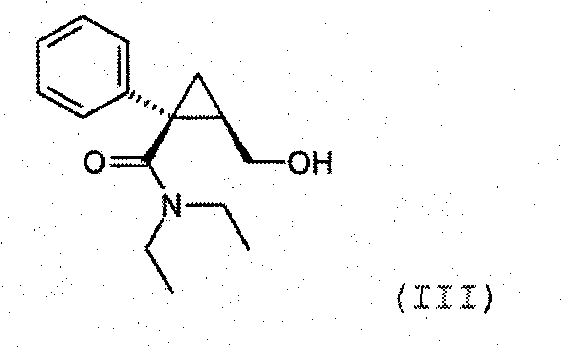
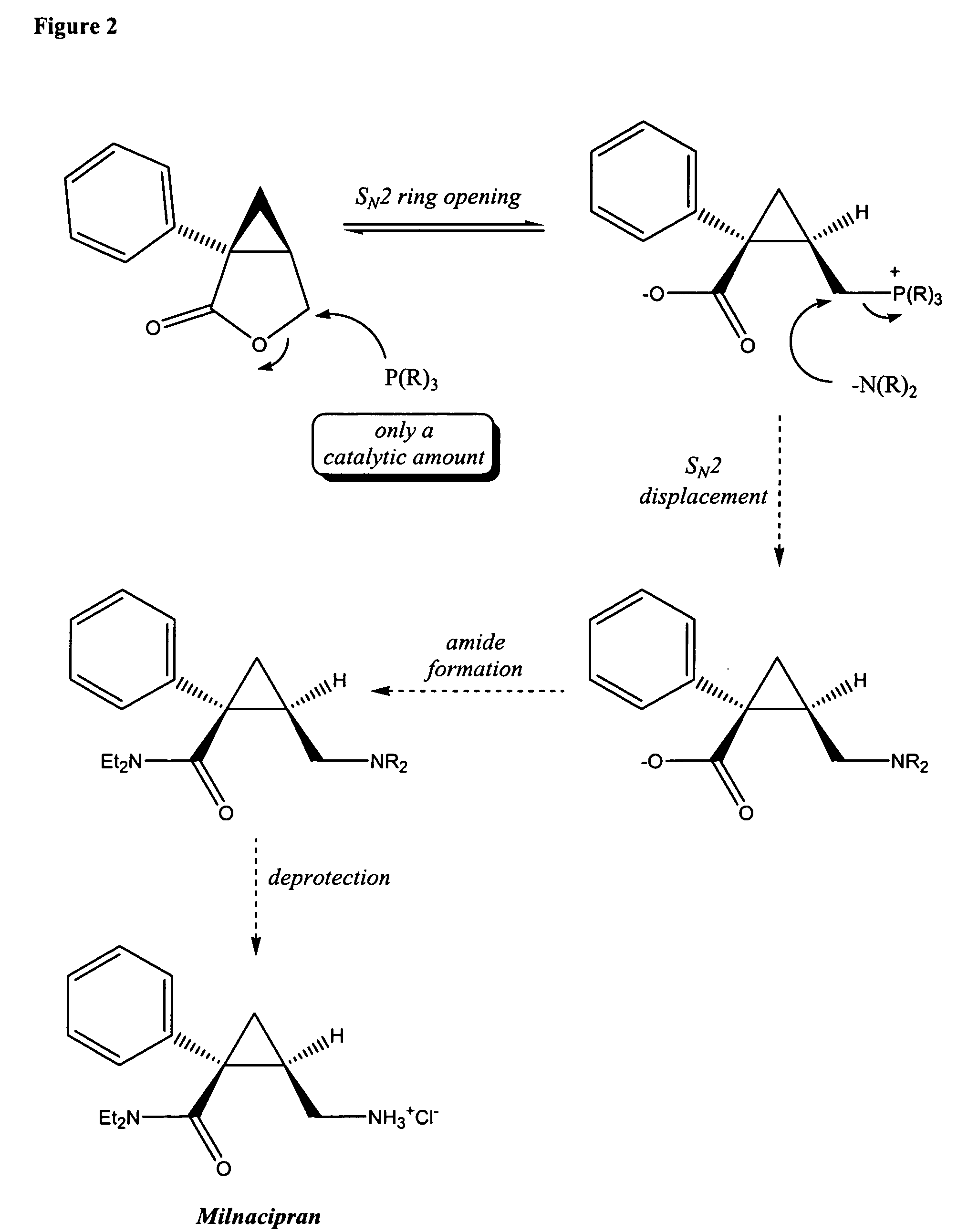
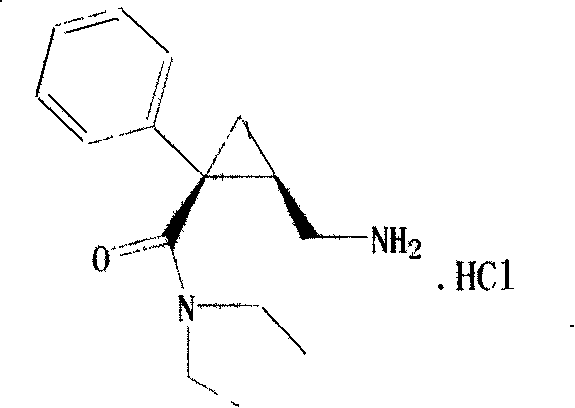
Synthesis of (1s,2r)-milnacipran
InactiveCN102300840AEasy to manufactureLow costCarboxylic acid amides optical isomer preparationAlcoholMedicinal chemistry
The present invention relates to the method for synthesizing the pharmaceutically acceptable acid addition salt of (1S,2R)-milnacipran, it comprises following successive steps: (a) phenylacetonitrile and (R)-epichlorohydrin Reaction under the condition that alkali exists, after alkali treatment, acid treatment, obtain lactone; (b) under the condition that Lewis acid-amine complex exists, this lactone and MNEt2 (M represents alkali metal) or NHEt2 reaction, obtain Amide alcohol; (c) the amide alcohol reacts with thionyl chloride to obtain amide chloride; (d) the chloride amide reacts with phthalimide salt to obtain phthalimide derivatives; (e) hydrolyzing the phthalimide group of the phthalimide derivative to obtain (1S, 2R)-milnacipran; (f) in the presence of a pharmaceutically acceptable acid (1S,2R)-milnacipran is salted in a suitable solvent system under the same conditions.
Owner:PIERRE FABRE MEDICAMENT SAS
Milnacipran for the treatment of cognitive dysfunction associated with fibromyalgia
InactiveUS20080058318A1Effective treatmentGood curative effectBiocideNervous disorderLong term treatmentsHigh doses
Methods for treating cognitive dysfunction associated with fibromyalgia by administering high-dose milnacipran to a patient suffering from such cognitive dysfunction are provided. Also provided are methods for the long-term treatment of cognitive dysfunction associate with FMS by administering milnacipran to a patient suffering from such cognitive dysfunction.
Owner:CYPRESS BIOSCI
Methods for the synthesis of milnacipran and congeners thereof
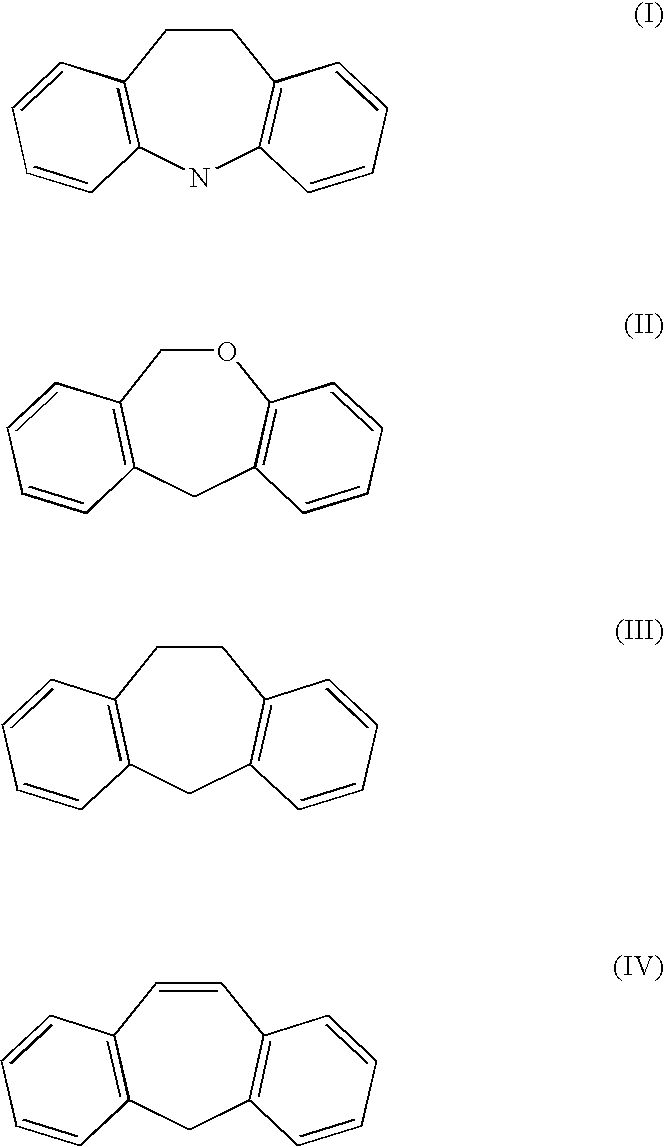
One aspect of the present invention relates to methods for synthesizing milnacipran or congeners thereof. Another aspect of the present invention relates to asymmetric methods for synthesizing enantiomerically enriched milnacipran or congeners thereof. The present invention also relates to methods for synthesizing intermediates useful in the non-asymmetric or asymmetric methods for synthesizing enantiomerically enriched milnacipran or congeners thereof.
Owner:COLLEGIUM PHARMA INC
Methods for improving physical function in fibromyalgia
Methods for improving physical function in fibromyalgia syndrome by administering an NSRI such as milnacipran as disclosed.
Owner:CYPRESS BIOSCI
Process for preparing optical pure milnacipran and its pharmaceutically accepted salts
ActiveUS20100016636A1Good yieldLow costNervous disorderOrganic compound preparationMedicinal chemistryMilnacipran
The present invention discloses a process for preparing optically pure milnacipran and their pharmaceutically acceptable salts, which adopts racemic milnacipran as starting material, tartaric acid derivatives and their compositions as resolving agents to resolve.
Owner:ZHEJIANG HAISEN PHARMACY CO LTD
Optics pure milnacipran and production method of its salt
InactiveCN101195583ALow costEasy to operateNervous disorderOrganic compound preparationMilnacipranChemistry
The invention discloses a method for preparing optical pure milnacipran and medicinally acceptable salt, which adopts racemic milnacipran as raw material, tartaric acid derivative and composition as resolution agent to resolve.
Owner:SICHUAN ANTIBIOTIC IND RES INST CO LTD
Modified release compositions of milnacipran
InactiveUS20100196472A1Diminished incidence and decreased intensityImprove sweatingOrganic active ingredientsBiocideMedicinal chemistryExcipient
A milnacipran formulation that provides delayed and extended release of milnacipran has been developed. The formulation comprises milnacipran or a salt thereof; an extended release excipient, and a delayed release excipient. The formulation provides, upon administration to a human subject, a Tmax of 4-10 hours.
Owner:COLLEGIUM PHARMA INC
Process for preparing optical pure milnacipran and its pharmaceutically accepted salts
ActiveUS8222454B2Good yieldLow costNervous disorderOrganic compound preparationMedicinal chemistryMilnacipran
The present invention discloses a process for preparing optically pure milnacipran and their pharmaceutically acceptable salts, which adopts racemic milnacipran as starting material, tartaric acid derivatives and their compositions as resolving agents to resolve.
Owner:ZHEJIANG HAISEN PHARMACY CO LTD
Process for the preparation of pharmaceutically acceptable salts of racemic milnacipran and its optical enantiomers thereof
InactiveUS20120184774A1Organic compound preparationCarboxylic acid amides optical isomer preparationEnantiomerMedicinal chemistry
The present invention relates to an improved process for the preparation of pharmaceutically acceptable salts of milnacipran by mutual acid radical exchange.
Owner:ARCH PHARMALABS LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Composition transferred through nose and brain containing milnacipran
InactiveCN101301283AImprove distributionQuick effectPowder deliveryOrganic active ingredientsWhole bodyNose
The invention provides a Milnacipran composition transferred through noses and brains, which contains a Milnacipran original drug or a pharmaceutically acceptable salt thereof and pharmaceutically necessary auxiliary materials. The composition transferred through the noses and the brains can target to a brain tissue, quickly take effect, reduce adverse reaction of the whole body, and is convenient to use.
Owner:SHANGHAI INST OF PHARMA IND
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
ActiveUS20140221388A1Effective treatmentNervous disorderAmine active ingredientsSertralineDuloxetine
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
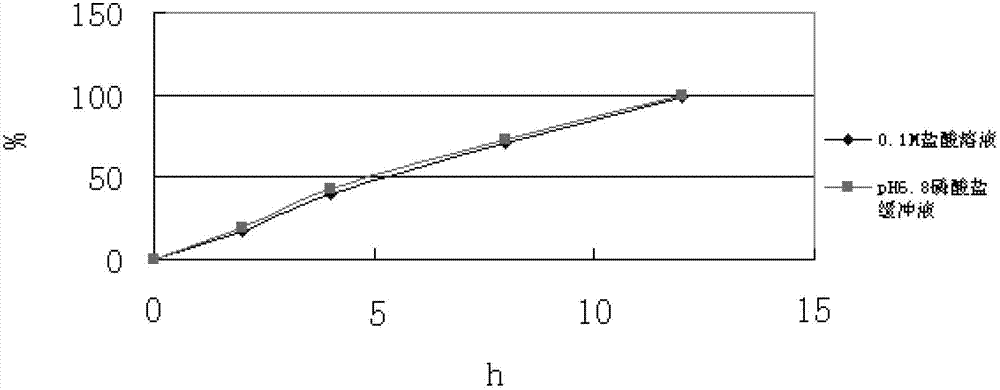
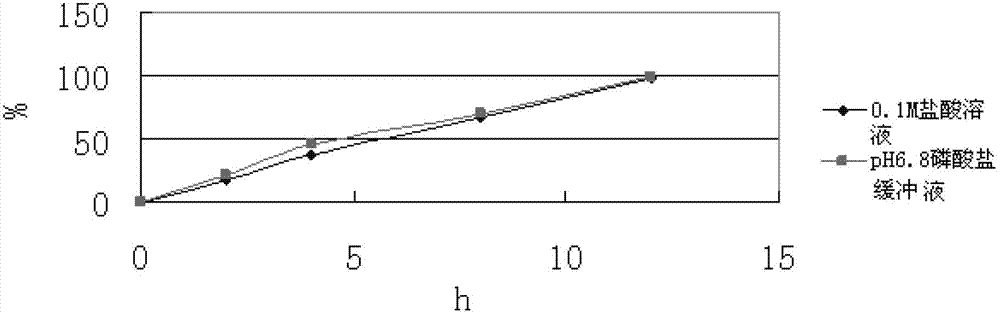
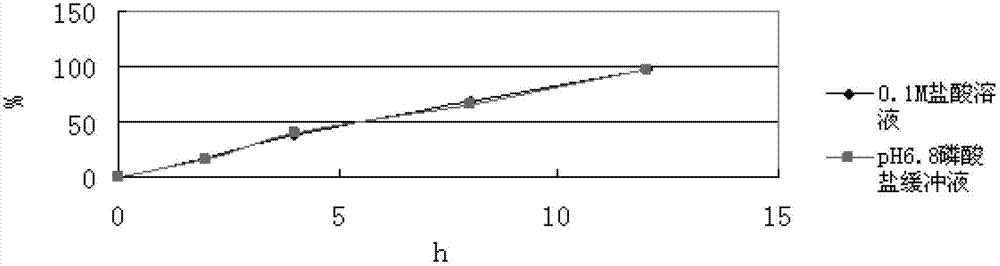
Slow-release composition containing L-milnacipran and preparation method thereof
ActiveCN103083274ARelease Behavior ConsistentReduce lossesOrganic active ingredientsNervous disorderAcrylic resinHypromellose
The invention relates to a slow-release composition containing L-milnacipran and a preparation method thereof. The composition contains the L-milnacipran serving as an active ingredient or pharmaceutically acceptable salt thereof. The composition is characterized by also comprising high-viscosity hydroxypropyl methylcellulose serving as a hydrophilic gel slow-release skeleton material and acrylic resin serving as a pH adjustor, wherein the composition comprises the following components in parts by weight: 10 to 70 parts of L-milnacipran or pharmaceutically acceptable salt, 5 to 30 parts of hydroxypropyl methylcellulose, and 3.5 to 7.5 parts of acrylic resin; and during preparation, the L-milnacipran, the hydroxypropyl methylcellulose and the acrylic resin are bound to be mixed uniformly. The composition can realize consistency of release behaviors in artificial gastric juice and artificial intestinal juice and keep 12-hour continuous release effect, and is low in preparation loss, high in efficiency, high in stability, easy to operate and easy to popularize.
Owner:葛亚伯
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com