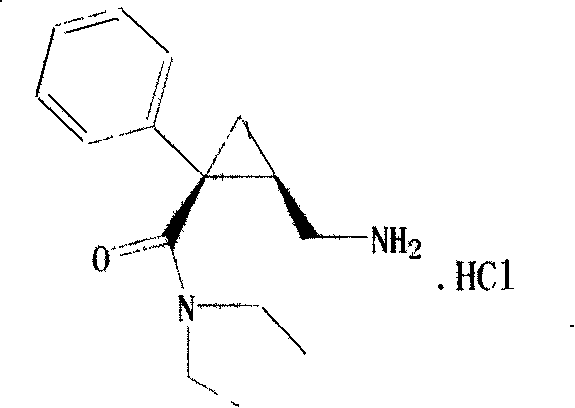
Optics pure milnacipran and production method of its salt
一种米那普仑、光学的技术,应用在米那普仑及其盐的制备领域,能够解决成本高、操作复杂等问题,达到成本低、操作简单、适合大规模生产使用的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
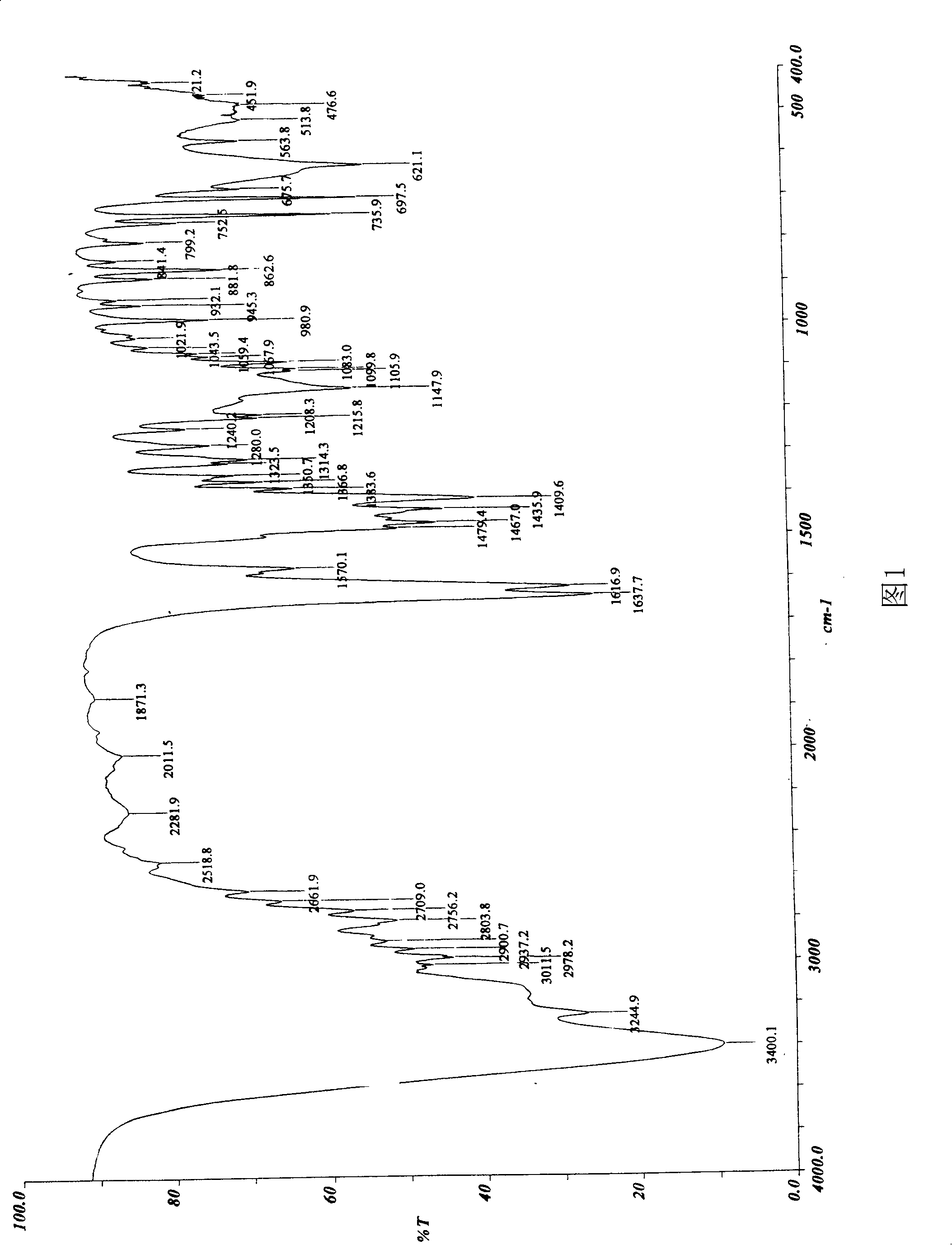
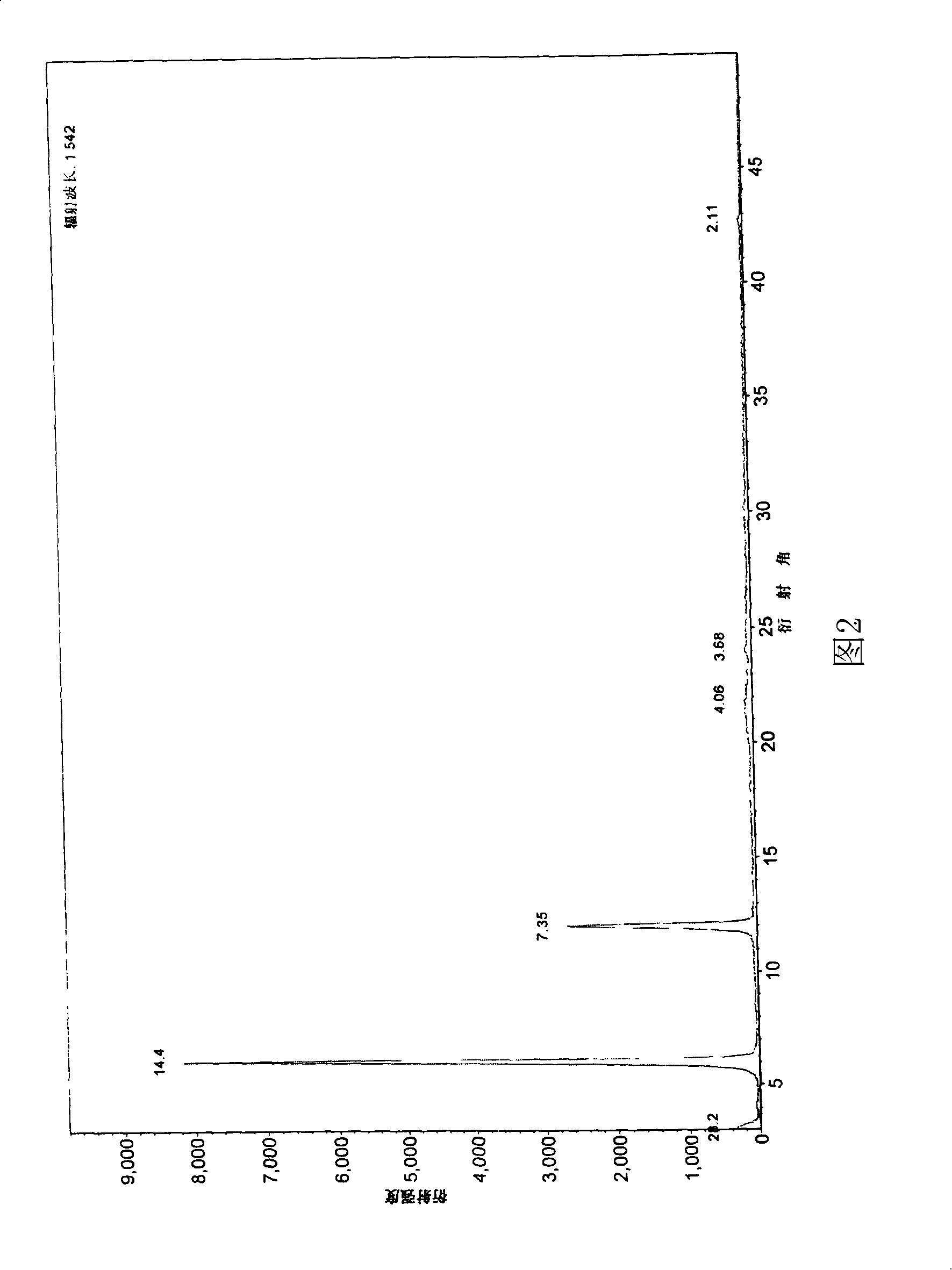
Image
Examples
Embodiment 1
[0031] Example 1 Resolution of dextrose milnacipran with D-resolving agent family T1
[0032] Suspend 5.000 g (17.65 mmol) of milnacipran hydrochloride in a mixture of 50 ml of aqueous solution and 50 ml of dichloromethane, and add 10% aqueous sodium hydroxide solution with stirring until the aqueous phase is alkaline (pH=11). Separate the organic phase, extract the aqueous phase with dichloromethane (40ml each time) for 3 times, combine the organic phases, wash the organic phase twice with saturated brine, then dry with anhydrous sodium sulfate, filter, and distill off the dichloromethane to obtain Racemic Milnacipran. The resulting racemic milnacipran and 7.10g (17.65mmol) D-resolving agent family T1 were dissolved in 225ml 98% acetone-water respectively, mixed, crystallized, filtered to obtain (+)-milnacipran and D-Resolution product 5.59 g, yield: 85.0% by resolving agent family T1, the optical purity of (+)-milnacipran contained in it is 99.8% e.e.
Embodiment 2
[0033] Embodiment 2 uses D-di-p-methoxybenzoyl tartaric acid to resolve cyclized milnacipran
[0034] With 5.000g (17.65mmol) vortex milnacipran hydrochloride, according to the method of embodiment 1, obtain racemic milnacipran. The obtained racemic milnacipran and 8.865g (17.65mmol) D-di-p-methoxybenzoyl tartaric acid were dissolved in 500ml 98% isopropanol-water respectively, mixed, crystallized, and filtered to obtain (+) - Milnacipran and D-di-p-methoxybenzoyl tartaric acid 5.66g resolution product, yield: 86.0%, the optical purity of (+)-milnacipran contained in it is 99.9% e.e.
Embodiment 3
[0035] Embodiment 3 uses L-resolving agent family T to resolve the cyclized milnacipran
[0036] With 1.000g (3.53mmol) mixed milnacipran hydrochloride, according to the method of embodiment 1, obtain racemic milnacipran. The resulting racemic milnacipran and 1.420g (3.53mmol) L-resolving agent family T were dissolved in 45ml 98% acetone-water respectively, mixed, crystallized, filtered to obtain (-)-milnacipran and The resolution product of L-resolving agent family T1 was 1.094 g, yield: 83.1%, and the optical purity of (-)-milnacipran contained in it was 69.0% e.e.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com