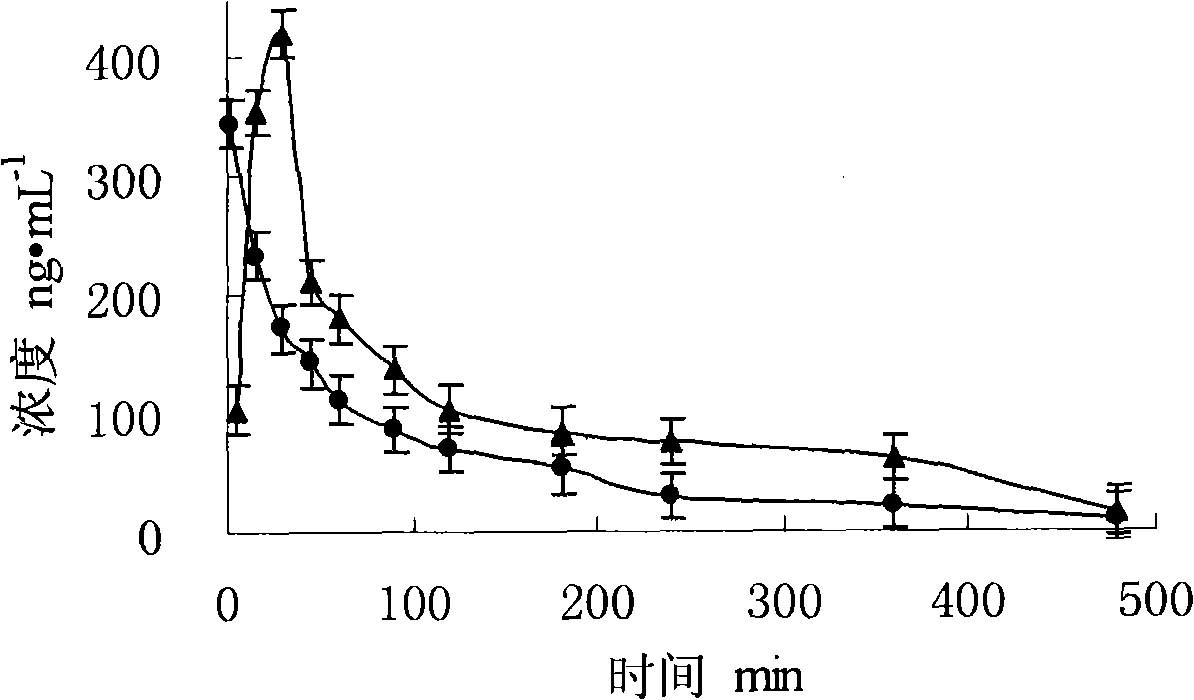
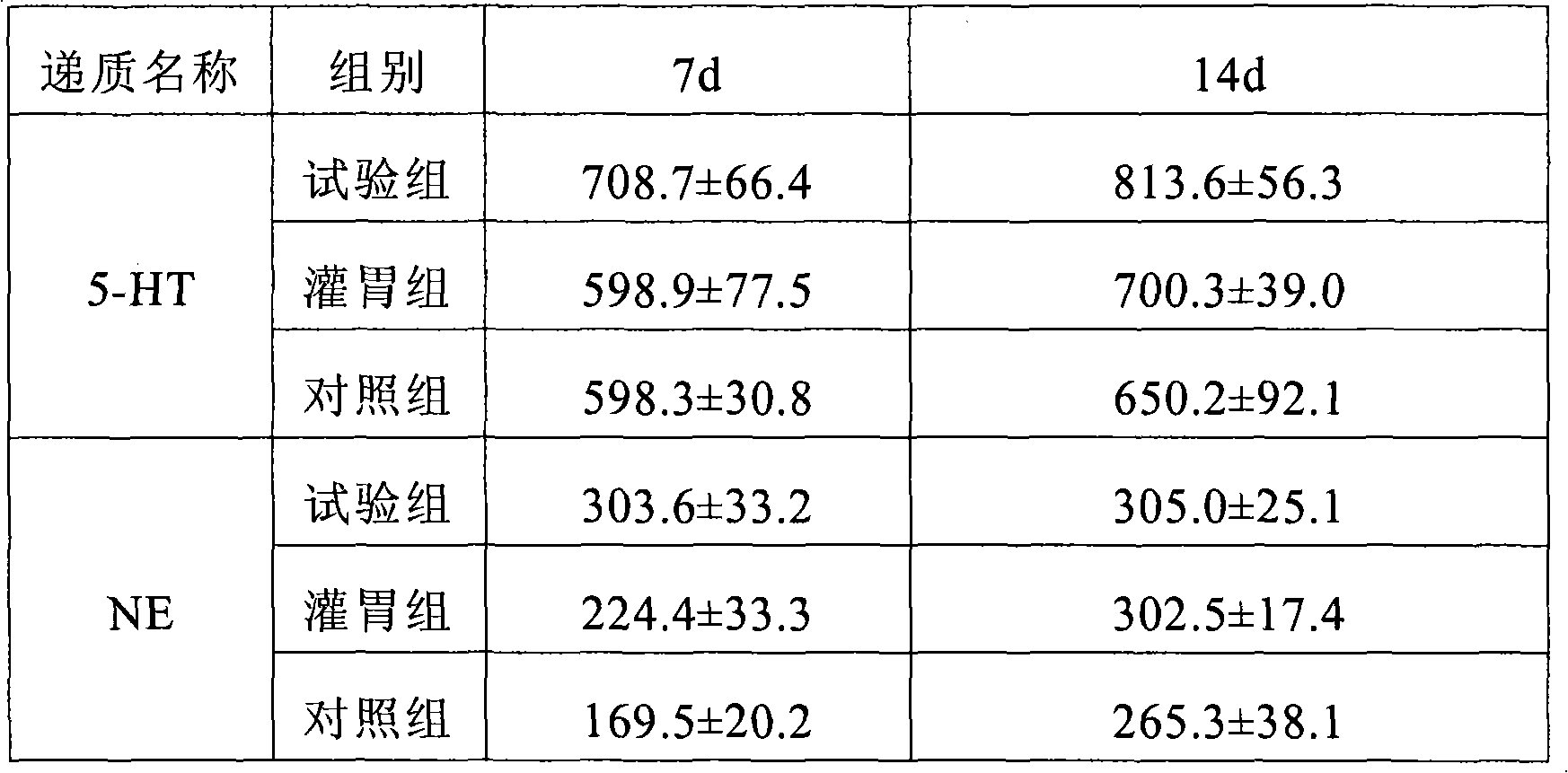
Composition transferred through nose and brain containing milnacipran
A technology of milnacipran and a composition, which is applied in the field of transnasal-brain transfer composition, can solve the problems of no central selectivity, peripheral adverse reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Prepare milnacipran nasal drops according to the following ratio:
[0083] Milnacipran
[0084] Preparation method: Take 800ml of deionized water, weigh the prescribed amount of milnacipran, sodium chloride, benzalkonium chloride, sodium carboxymethylcellulose, and disodium edetate, stir and heat to dissolve them completely , after cooling, add deionized water to settle to 1000ml, and adjust the pH to 7 with 0.1mol / L hydrochloric acid to obtain milnacipran nasal drops.
Embodiment 2
[0086] Prepare milnacipran nasal drops according to the following ratio:
[0087] Milnacipran
[0088] Preparation method: take 800ml of deionized water, weigh the prescribed amount of milnacipran, benzalkonium chloride, sodium carboxymethylcellulose, and disodium edetate, stir and heat to dissolve completely, after cooling, Add deionized water to make the volume to 1000ml, and adjust the pH to 7 with 0.1mol / L hydrochloric acid to obtain milnacipran nasal drops.
Embodiment 3
[0090] Prepare milnacipran nasal drops according to the following ratio:
[0091] Milnacipran
[0092] Sodium carboxymethyl cellulose
[0093] Preparation method: Take 800ml of deionized water, weigh the prescribed amount of milnacipran, sodium chloride, benzalkonium chloride, sodium carboxymethylcellulose, and disodium edetate, stir and heat to dissolve them completely , after cooling, add deionized water to settle to 1000ml, and adjust the pH to 7 with 0.1mol / L hydrochloric acid to obtain milnacipran nasal drops.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com