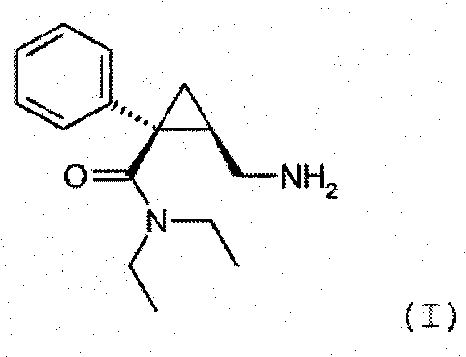
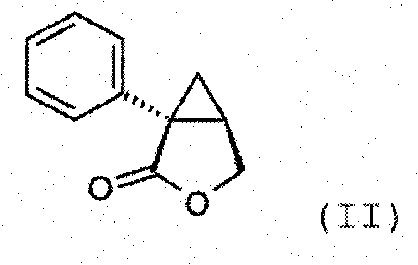
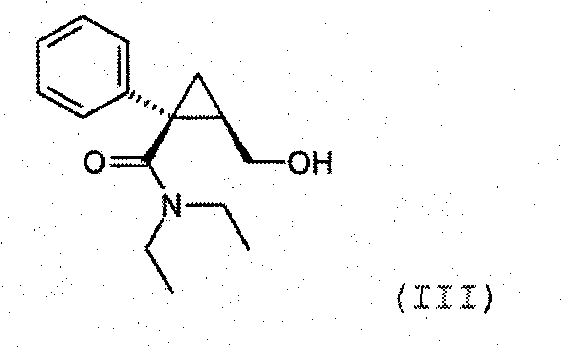
Synthesis of (1s,2r)-milnacipran
A technology of milnacipran and general formula, applied in the field of asymmetric synthesis of chlorinated intermediates, can solve the problems of toxic explosion, regardless of industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0073] Synthesize the final product (1S, 2R)-milnacipran hydrochloride with a total weight of 41kg according to the following scheme and operation process:
[0074]
[0075] Steps 1 to 4:
[0076] 28 kg of sodium amide (682 moles) was suspended in 400 L of toluene, then 85.5 kg of phenylacetonitrile (729.5 moles) diluted in 10 L of toluene was poured into the above mixture at a temperature ranging from 0°C to 5°C under vigorous stirring. The reaction medium is stirred at 10° C. for at least 1 h. Maintaining the reaction temperature at 10°C, 27 kg of chiral epichlorohydrin (292 moles) dissolved in 20 L of toluene was added. After pouring is complete, the medium is stirred for at least 2 h. Maintain the reaction temperature between 5°C and 40°C, pour the reaction medium into 240L aqueous solution to carry out the hydrolysis reaction. After the resulting solution was concentrated, 115 kg of 30% sodium carbonate was added to the medium, followed by heating to 95° C. to hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com