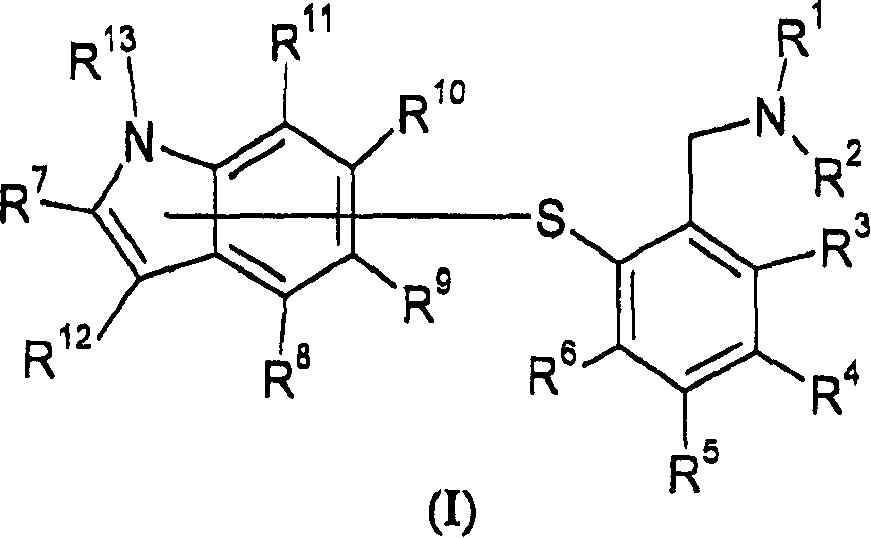
2-(1h-indolylsulfanyl)-benzyl amine derivatives as ssri
A technology based on sulfanyl and indole, applied in the field of norepinephrine reuptake inhibitors, can solve problems such as impossible progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0694] Another aspect of the present invention relates to the following processes for the preparation of the compounds of the present invention.
[0695] The preparation method of the compound of the present invention
[0696] The preparation of the compound of the present invention is as follows:
[0697] Method 1 (for compounds of formula IA), alkylation of amines of formula III with alkylated derivatives of formula II:
[0698]
[0699] where R 1 -R 13 As defined herein, L is a leaving group such as, for example, halogen, mesylate or tosylate;
[0700] The compound of formula IA is thus isolated as a free base or a salt thereof such as a pharmaceutically acceptable acid addition salt.
[0701] Method 2 (for compounds of formula IA), reduction of amide derivatives of formula IV below,
[0702]
[0703] where R 1 -R 13 as defined herein;
[0704] The compound of formula IA is thus isolated as a free base or a salt thereof such as a pharmaceutically acceptable ac...
Embodiment 12
[0765] Example 12-(1H-indol-3-ylsulfanyl)-N,N-dimethylbenzamide
[0766] N,N,N',N'-tetramethyl-2,2'-dithiobenzamide (Elworthy, Todd R.; Ford, Anthony P.D.W.; Bantle, Gary W. ; Morgans, David J.; Ozer, Rachel S.; et al. J.Med.Chem.40, 1997, 2674-2687) (12.80 g, 35.5 mmol) dissolved in 1,2-dichloroethane (200 mL), Sulfonyl chloride (2.9 mL, 4.84 g, 35.9 mmol) was added carefully under argon with stirring, the reaction mixture was stirred at room temperature for 15 min, and the resulting solution was added slowly (dropwise) under argon over ice Cooled (0° C.) solution of indole (8.4 g, 71.7 mmol) in anhydrous DMF (180 mL). The mixture was stirred at 0 °C under Ar for 2.5 h, then water (180 mL) and saturated NaHCO were added 3 The aqueous solution (150 mL) was quenched, and to the resulting emulsion was added ethyl acetate (250 mL). The organic phases were combined and washed with brine (100 mL). The aqueous phase was further extracted with ethyl acetate (2×100 mL), the combin...
Embodiment 2
[0791] 2-(1H-Indol-3-ylsulfanyl)-N-methylbenzamide
[0792] Carbonyldiimidazole (11 mmol) was added to 2-(1H-indol-3-ylsulfanyl)benzoic acid (Hamel, P.; Girard, M.; Tsou, N.N.; J. Heterocycl. Chem. 36, 1999 , 643-652) (10 mmol) in anhydrous THF (200 mL) and refluxed under argon for 60 minutes, methylamine (1M in THF; 40 mL) was slowly added to the reaction mixture, The mixture was stirred at room temperature for 16 hours, the mixture was evaporated in vacuo and the product was purified by column chromatography on silica gel using ethyl acetate as eluent.
[0793] The following compounds were prepared in a similar manner:
[0794] 2-(5-Fluoro-1H-indol-3-ylsulfanyl)-N-methylbenzamide
[0795] 2-(6-Fluoro-1H-indol-3-ylsulfanyl)-N-methylbenzamide
[0796] 2-(2-Methyl-1H-indol-3-ylsulfanyl)-N-methylbenzamide
[0797] 2-(4-Methyl-1H-indol-3-ylsulfanyl)-N-methylbenzamide
[0798] 2-(4-Chloro-1H-indol-3-ylsulfanyl)-N-methylbenzamide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com