A kind of levetiracetam sustained-release tablet and preparation method thereof
A technology of sustained-release tablet and sustained-release layer, which is applied in the field of levetiracetam sustained-release tablet and its preparation, and can solve the problems of ineffectiveness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
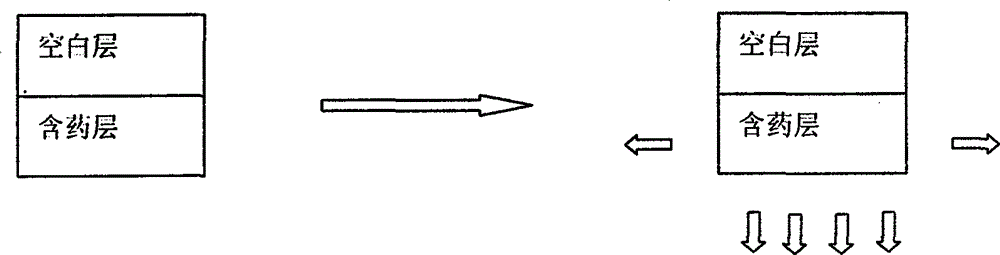
[0031] First, put the raw materials and auxiliary materials at 60°C for 4 hours, pass through an 80-mesh sieve for later use; then, prepare a blank layer: take 200 g of glyceryl behenic acid and 15 g of polyvinylpyrrolidone K3015 g and mix them uniformly in equal increments. Granulation by method, weighing; Preparation of drug-containing sustained-release layer: levetiracetam 500g, glyceryl behenate 150g, polyvinylglycerol 150g, polyvinylpyrrolidone K3020g are mixed with equal increments, dry granulation, Weighing; tableting: add 1% magnesium stearate to the blank layer and the drug-containing layer and mix them evenly, separate them into two hoppers and compress them into 1000 tablets on a double-layer tablet press. Each tablet contains 500mg of levetiracetam.
Embodiment 2
[0033] First, put the raw materials and auxiliary materials at 60°C and dry them for 4 hours, pass through an 80-mesh sieve for later use; then, prepare a blank layer: take 300 g of ethyl cellulose and 15 g of polyvinylpyrrolidone K3015 g and mix them uniformly in equal increments, and wet-process Granules, weighed; preparation of drug-containing sustained-release layer: levetiracetam 500g, ethyl cellulose 200g, polyvinylpyrrolidone K3020g were mixed in equal increments, wet granulated, weighed; tabletting: the blank Add 1% magnesium stearate to the layer and the drug-containing layer and mix evenly, separate the two hoppers and compress it into 1000 tablets on a double-layer tablet press. Each tablet contains 500mg of levetiracetam.
Embodiment 3
[0035] First, put the raw materials and auxiliary materials at 60°C for 4 hours, pass through an 80-mesh sieve for later use; then, prepare a blank layer: take 180 g of hypromellose and 20 g of polyvinylpyrrolidone K3020 g and mix them uniformly in an equal increment method, dry Granulation by method, weighing; preparation of drug-containing sustained-release layer: levetiracetam 500g hypromellose 150g, polyvinylpyrrolidone K30 20g were mixed with the same amount increasing method, dry granulation, weighing; tabletting: the The blank layer and the drug-containing layer are respectively added with 1% magnesium stearate and mixed evenly, separated into two hoppers and compressed into 1000 tablets on a double-layer tablet press. Each tablet contains 500mg of levetiracetam.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com