Pharmaceutical composition containing a statin in the form of a zinc salt
A composition and medicine technology, applied in the field of medicine, can solve the problems of poor stability, high production cost, complicated calcium salt production process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
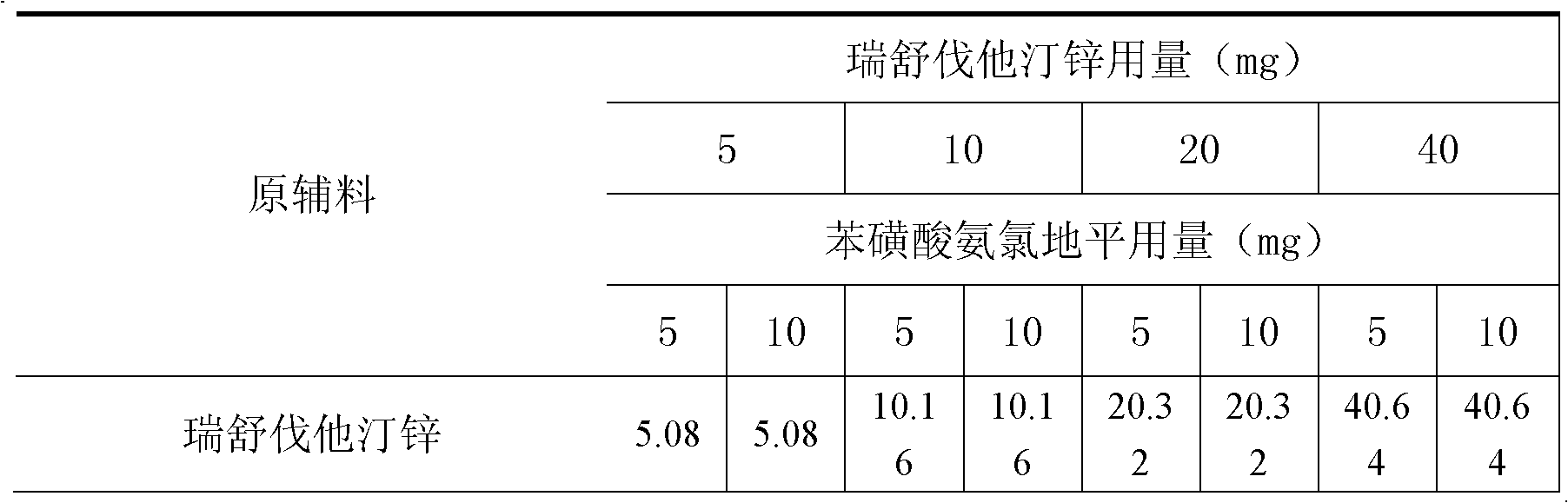
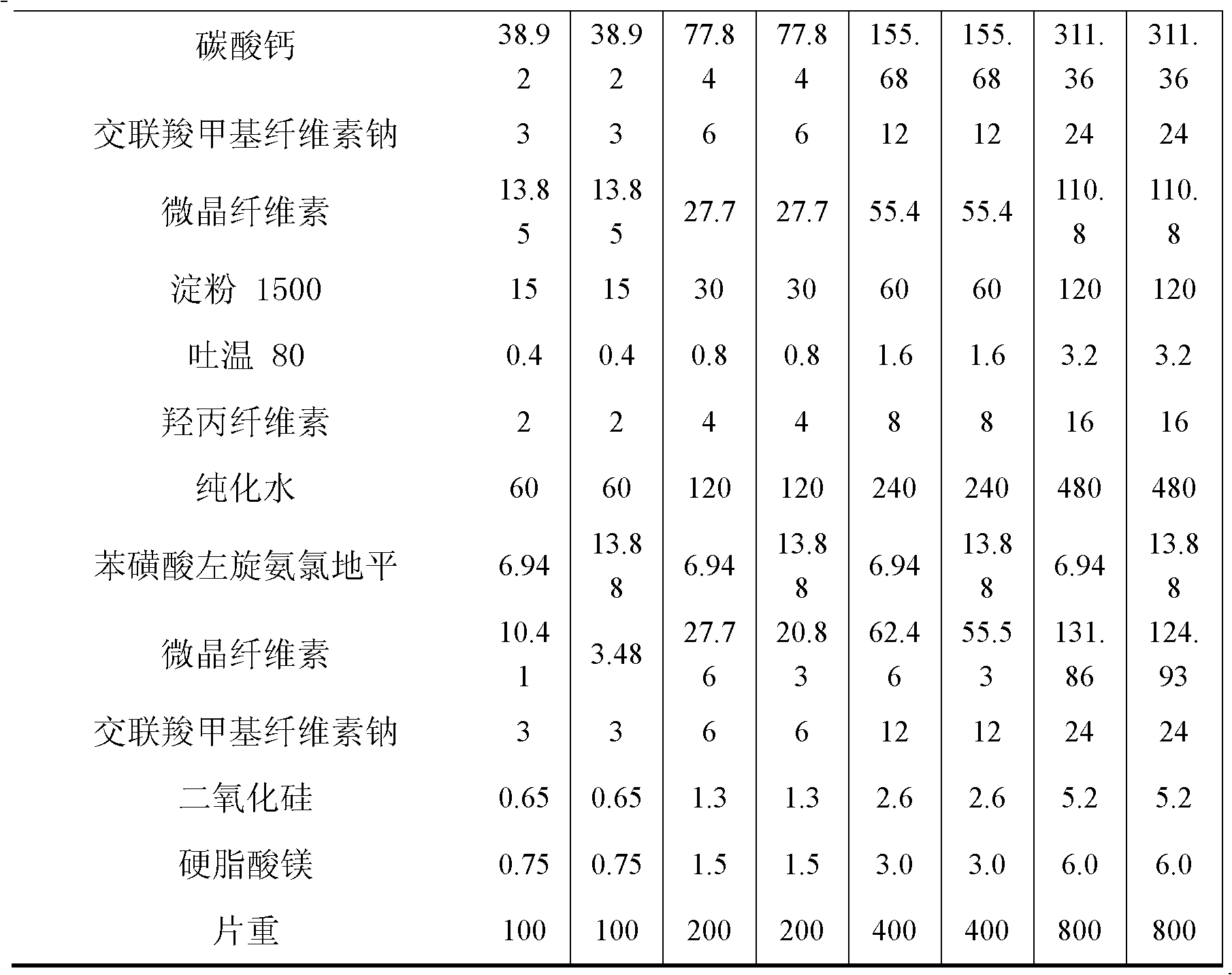
[0009] Example 1 Compound Rosuvastatin Zinc Amlodipine Besylate Tablets
[0010]
[0011]
[0012] Preparation:
[0013] 1. Add the prescribed amount of Tween 80 to purified water at 50°C, stir well to dissolve, add hydroxypropyl cellulose to hydrate it, and form a binder.
[0014] 2. The prescribed amount of rosuvastatin zinc, calcium carbonate, microcrystalline cellulose, starch 1500, and croscarmellose sodium are fully mixed uniformly by equal amount addition method.
[0015] 3. Add the binder soft material obtained in step 1 to the mixed powder obtained in step 2, and granulate with an 18-mesh sieve.
[0016] 4. Dry the granules obtained in step 3, and control the moisture content to be no more than 2.0%.
[0017] 5. Add levamlodipine besylate, microcrystalline cellulose, croscarmellose sodium, and silicon dioxide to the granules obtained in step 4 and mix well. Pass through a 24-mesh sieve for granulation.
Embodiment 2
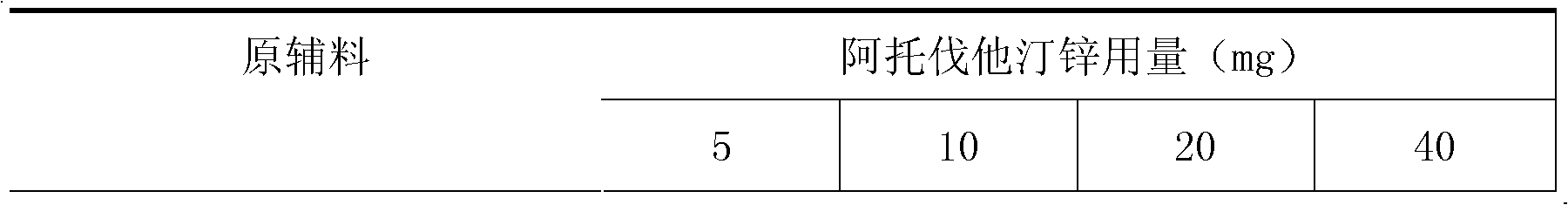
[0019] Example 2 Compound Atorvastatin Zinc Amlodipine Besylate Capsules
[0020]
[0021]
[0022] Preparation:
[0023] 1. Add the prescribed amount of Tween 80 to purified water at 50°C, stir well to dissolve, add hydroxypropyl cellulose to hydrate it, and form a binder.
[0024] 2. Fully mix the prescribed amount of atorvastatin zinc, calcium carbonate, pregelatinized starch, starch 1500, and sodium carboxymethyl starch by equal addition method.
[0025] 3. Add the binder soft material obtained in step 1 to the mixed powder obtained in step 2, and granulate with an 18-mesh sieve.
[0026] 4. Dry the granules obtained in step 3, and control the moisture content to be no more than 2.0%.
[0027] 5. Add amlodipine besylate, pregelatinized starch, sodium carboxymethyl starch, and silicon dioxide to the granules obtained in step 4 and mix well. Pass through a 24-mesh sieve for granulation.
[0028] 6. Add magnesium stearate to the mixed granules obtained in step 5, mi...
Embodiment 3
[0029] Example 3 Compound Rosuvastatin Zinc Nilvadipine Dispersible Tablets
[0030]
[0031]
[0032] Preparation:
[0033] First crush the raw materials through a 100-mesh sieve, fully mix the raw material drug, microcrystalline cellulose, and hydroxypropyl cellulose, add an appropriate amount of water-based soft material, granulate with a 18-mesh sieve, and dry at 60°C for 3-4 hours. Sieve through a 24-mesh sieve, add disintegrants croscarmellose sodium, aspartame, silicon dioxide, and magnesium stearate, mix well, and press into dispersible tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com