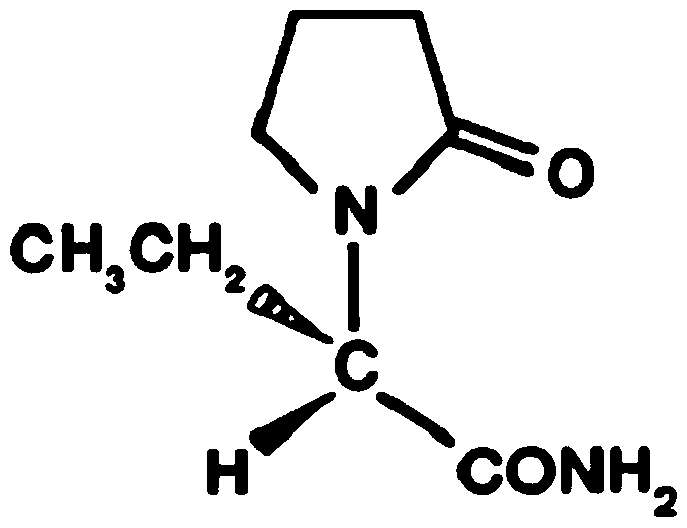
Method for preparing levetiracetam
An ethyl acetate, equivalent technology, applied in organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of poor product purity chromatographic purity, complex process, low step yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: preparation levetiracetam
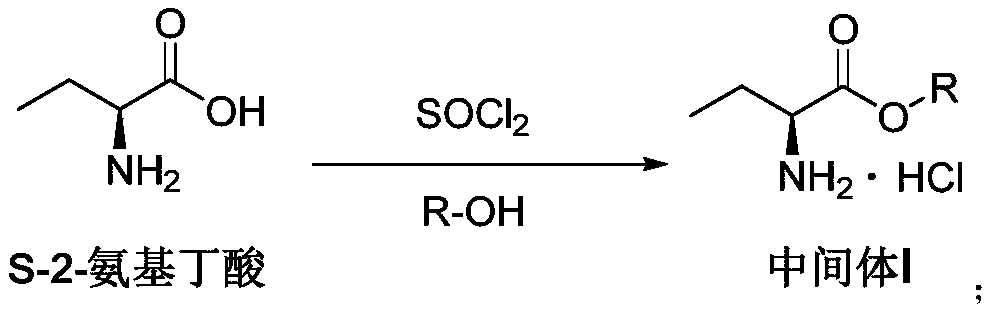
[0081] (1) Suspend 0.5 mol of (S)-2-aminobutyric acid in 12 equivalents of methanol in a reaction flask, add 1.2 equivalents of the reaction solvent thionyl chloride dropwise at a temperature of 25°C, and slowly raise the temperature after the addition is completed To 45°C, keep stirring for 4 hours to complete the reaction, concentrate under reduced pressure at 45°C to remove the solvent, add 2 equivalents of reaction solvent after the product is viscous, stir and cool down to -2-2°C to form a suspension.
[0082] (if not specified otherwise, the material represented by equivalent weight in the present invention all refers to the relative molar amount compared with the starting material of this step)
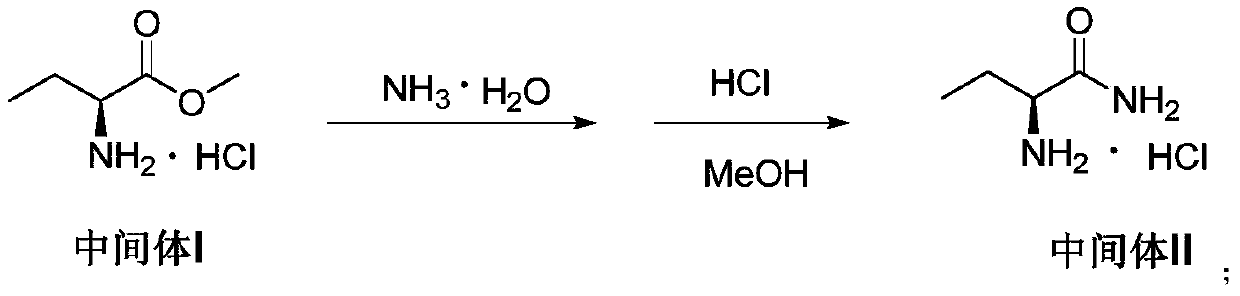
[0083] (2) Slowly add 8 equivalents of cold (0°C) ammonia water and 0.2 equivalents of oleic acid to the reaction flask of the suspension obtained in the previous step, stir for 6 hours, heat up to 20°C and continue stirring fo...
Embodiment 2
[0087] Embodiment 2: preparation levetiracetam
[0088] (1) Suspend 0.5 mol of (S)-2-aminobutyric acid in 12 equivalents of methanol in a reaction flask, add 1.2 equivalents of the reaction solvent thionyl chloride dropwise at a temperature of 30°C, and slowly raise the temperature after the addition is completed to 50°C, keep stirring for 4 hours to complete the reaction, concentrate under reduced pressure at 40°C to remove the solvent, add 2 equivalents of reaction solvent after the product is viscous, stir and cool down to -1-5°C to form a suspension.
[0089] (2) Slowly add 6 equivalents of cold (0°C) ammonia water and 0.3 equivalents of oleic acid into the reaction flask of the suspension obtained in the previous step, stir to react for 7 hours, heat up to 20-25°C and continue stirring for 3 hours to complete the reaction, add 0.2 equivalents of ethanol, 85 ° C under reduced pressure to distill ammonia water until a large amount of product precipitates, then add 6 equiv...
Embodiment 3
[0093] Embodiment 3: preparation levetiracetam
[0094] (1) Suspend 0.5 mol of (S)-2-aminobutyric acid in 12 equivalents of methanol in a reaction flask, add 1.2 equivalents of the reaction solvent thionyl chloride dropwise at a temperature of 20°C, and slowly raise the temperature after the addition is completed to 40°C, keep stirring for 4 hours to complete the reaction, concentrate under reduced pressure at 50°C to remove the solvent, add 2 equivalents of reaction solvent after the product is viscous, stir and cool down to -5-1°C to form a suspension.
[0095] (2) Slowly add 10 equivalents of cold (0°C) ammonia water and 0.1 equivalents of oleic acid into the reaction flask of the suspension obtained in the previous step, stir for 5 hours, heat up to 15-20°C and continue stirring for 4 hours to complete the reaction, add 0.2 equivalents of ethanol, 85 ° C under reduced pressure to distill ammonia water until a large amount of product precipitates, then add 6 equivalents o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com