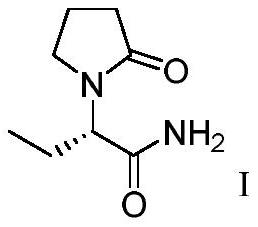
Preparation method of levetiracetam intermediate
A technology of ethyl and acetic acid, applied in the field of medicine and chemical industry, can solve the problems of residual raw materials, purity less than 80%, unstable yield, etc., and achieve the effect of inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
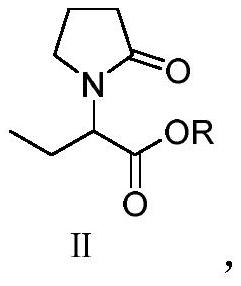
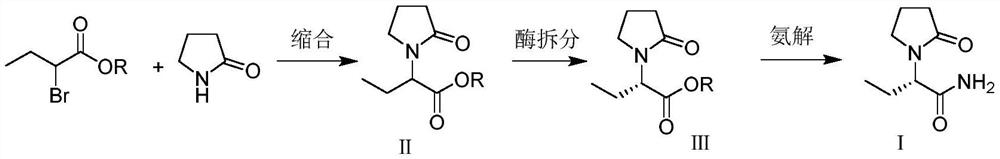
[0034] Preparation of α-ethyl-2-oxo-1-pyrrolidine acetate (II)
[0035] Add α-ethyl-2-oxo-1-pyrrolidineacetic acid (50g, 0.29mol) and methanol (100g) into the reaction flask, add concentrated sulfuric acid (2g) dropwise, and heat up to 65-70°C under stirring to reflux for 2 hour, add water (100g), control the temperature in the range of 40-50°C, add 10% Na dropwise under stirring 2 CO 3 Solution, until the pH of the reaction system is 7.0 to 7.5, control the temperature at 40 to 50°C and distill off the remaining methanol under reduced pressure, extract the residual solution twice with toluene (100g), combine the organic phases, and distill under reduced pressure until no liquid flows out to obtain α- Ethyl-2-oxo-1-pyrrolidine acetate, yield 49.1 g, yield 90.7%, HPLC purity 99.2%.
Embodiment 2
[0037] Preparation of α-ethyl-2-oxo-1-pyrrolidine acetate (II)
[0038] Add α-ethyl-2-oxo-1-pyrrolidineacetic acid (50g, 0.29mol) and methanol (100g) into the reaction flask, add concentrated sulfuric acid (4g) dropwise, and heat up to 65-70°C under stirring for 3 hour, add water (100g), control the temperature in the range of 40-50°C, add 10% NaHCO dropwise under stirring 3 Solution, until the pH of the reaction system is 7.0 to 7.5, the temperature is controlled at 40 to 50°C and the remaining methanol is evaporated under reduced pressure, and the residual solution is extracted twice with ethyl acetate (100g), the organic phases are combined, and distilled under reduced pressure until no liquid flows out to obtain α-Ethyl-2-oxo-1-pyrrolidine acetate, yield 49.3g, yield 91.2%, HPLC purity 99.4%.
Embodiment 3
[0040] Preparation of α-ethyl-2-oxo-1-pyrrolidine acetate (II)
[0041] Add α-ethyl-2-oxo-1-pyrrolidineacetic acid (50g, 0.29mol) and ethanol (150g) into the reaction flask, add concentrated sulfuric acid (5g) dropwise, and heat up to 78-80°C under stirring for 2 hour, add water (100g), control the temperature in the scope of 40~60 ℃, add dropwise 15%Na 2 CO 3 solution until the pH of the reaction system is 7.0 to 7.5, the remaining ethanol is evaporated under reduced pressure at 40 to 50°C under temperature control, the residual liquid is extracted 3 times with dichloromethane (100g), the organic phases are combined, and distilled under reduced pressure until no liquid flows out to obtain α-Ethyl-2-oxo-1-pyrrolidine ethyl acetate, yield 52.4g, yield 90.1%, HPLC purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com