Sodium valproate sustained release tablet as well as preparation process and application thereof
A technology of sodium valproate and valproic acid, which is applied in non-active ingredients medical preparations, anhydride/acid/halide active ingredients, nervous system diseases, etc. Poor uniformity and other problems, to achieve the effect of less type and dosage of excipients, low equipment requirements, and stable drug release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
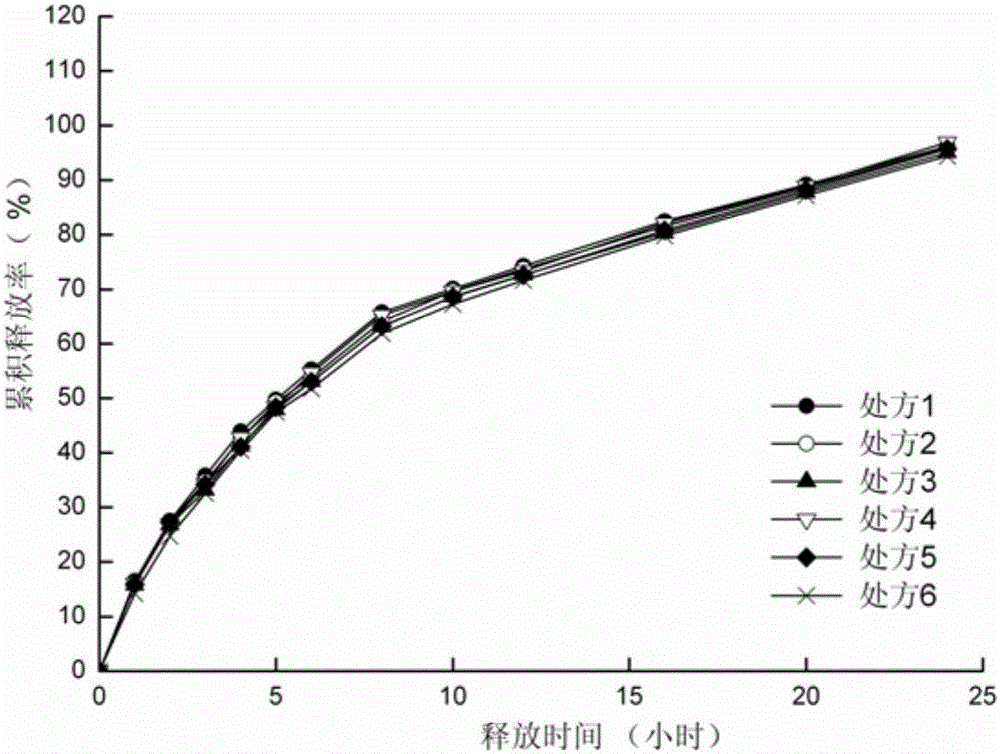
[0041]Preparation process: mix sodium valproate, hydroxypropylmethylcellulose and microcrystalline cellulose through a 60-mesh sieve according to the dosage in Table 1. Add liquid valproic acid and 95% ethanol to the above mixture to make a soft material, pass through a 20-mesh sieve to make wet granules, dry at 60°C for 2 hours, and sizing the granules with a 20-mesh sieve. The obtained granules are added with micropowder silica gel and a lubricant, mixed evenly, and compressed into tablets to obtain slow-release tablets.
[0042] Table 1 Sodium valproate sustained-release tablets (1000 tablets)
[0043]
[0044] In vitro release assay:
[0045] The first method of release measurement method in appendix XD of the Chinese Pharmacopoeia 2010 edition was selected, using 900ml phosphate buffer solution (pH6.8) as the release medium, and the rotation speed was 60 rpm, at the predetermined time points (1, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24h) samples were taken to determine t...
Embodiment 2
[0050] Adopt the sodium valproate slow-release tablet among the embodiment 1 to carry out stability study, investigate the sodium valproate slow-release tablet that makes with this prescription process at 40 ℃ ± 2 ℃, under the condition of relative humidity 75% ± 5% Placed for 6 months of stability. Measured by the items in Table 9 in the 0th, 1st, 2nd, 3rd, and 6th months respectively, the measurement results are the mean values of the measurement results of the samples selected under each prescription in Example 1.
[0051] Table 3 Example 1 Prescription Stability Investigation
[0052]
[0053] The above stability test results show that the slow-release tablet prepared according to the prescription in Example 1 has been subjected to an accelerated test for 6 months, and all indicators are up to the standard, and its stability meets the requirements.
Embodiment 3
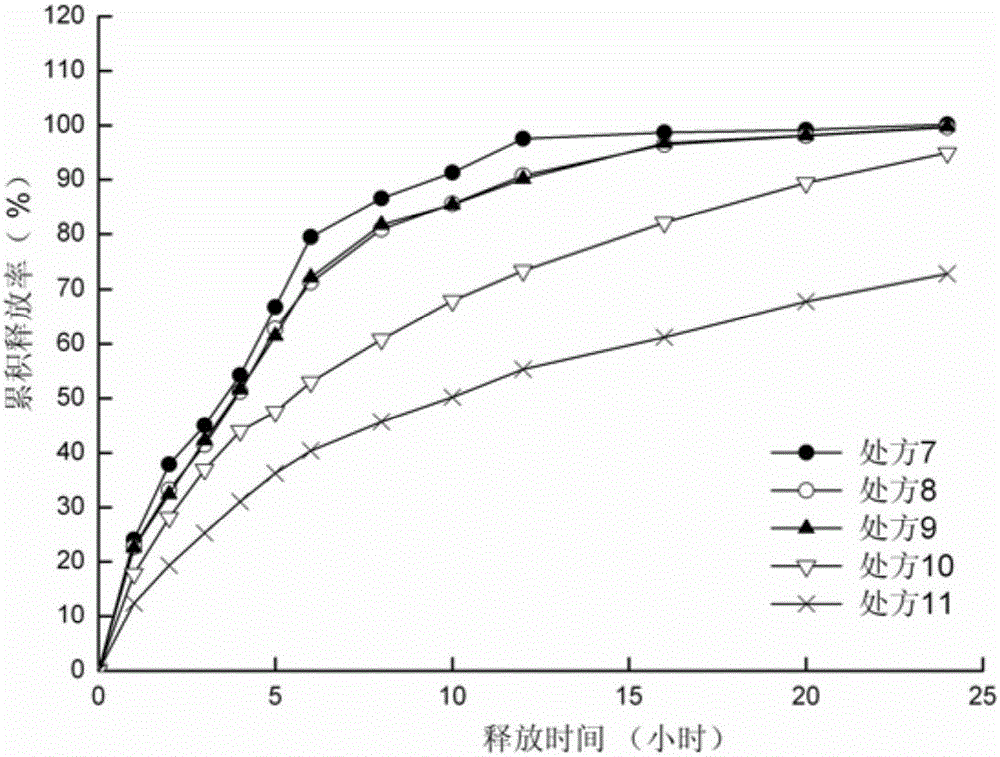
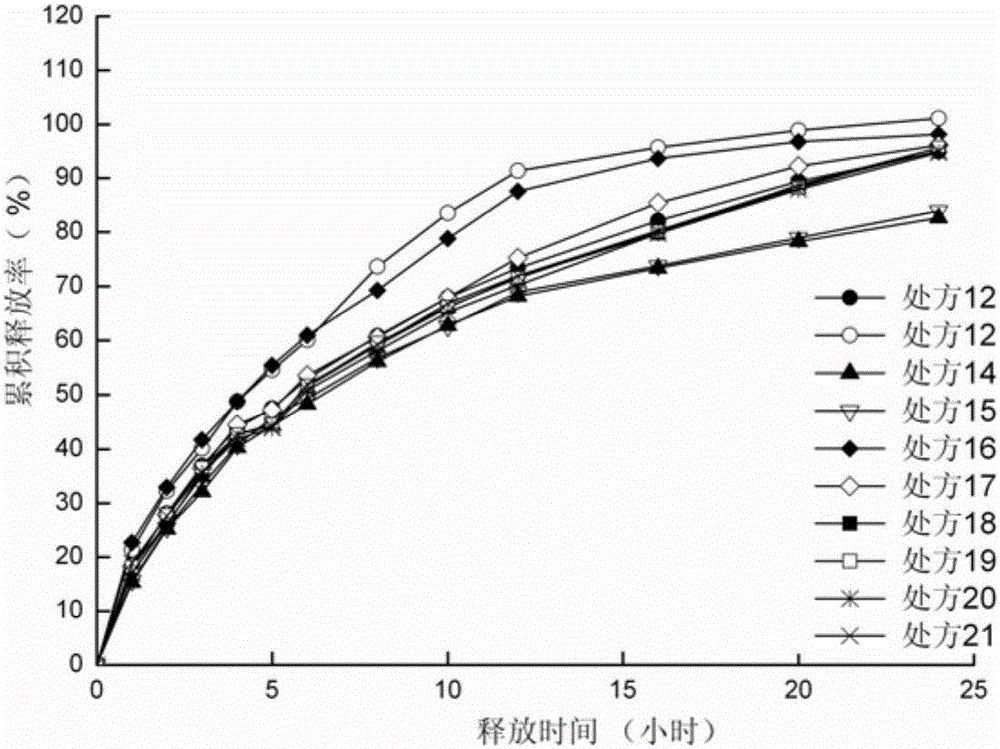
[0055] Preparation process: According to the dosage in Table 4 and Table 7, sodium valproate, blocking agent and filler are passed through a 60-mesh sieve for mixing. Add liquid valproic acid and 95% ethanol to the above mixture to make a soft material, pass through a 20-mesh sieve to make wet granules, dry at 60°C for 2 hours, and sizing the granules with a 20-mesh sieve. The obtained granules are added with micropowder silica gel and magnesium stearate, mixed evenly, and compressed into tablets to obtain slow-release tablets.
[0056] Table 4 Prescription of Sodium Valproate Sustained Release Tablets (1000 Tablets)
[0057] prescription
7
8
9
10
11
Sodium valproate (g)
333
333
333
333
333
Valproic acid (g)
145
145
145
145
145
HPMC E5 (g)
-
-
-
-
-
HPMC K4M (g)
100
-
-
-
-
HPMC K10M (g)
-
100
-
-
-
HPMC K15M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com