New crystal form for sodium valproate and preparation method and usage thereof
A technology of sodium valproate and crystal form, applied in the field of new crystal form of sodium valproate, can solve the problems of incomplete dissolution, loose control of endotoxin index, slow dissolution of drugs, etc., to ensure safety and stability, The effect of reducing the risk of pyrogen reaction, good clinical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
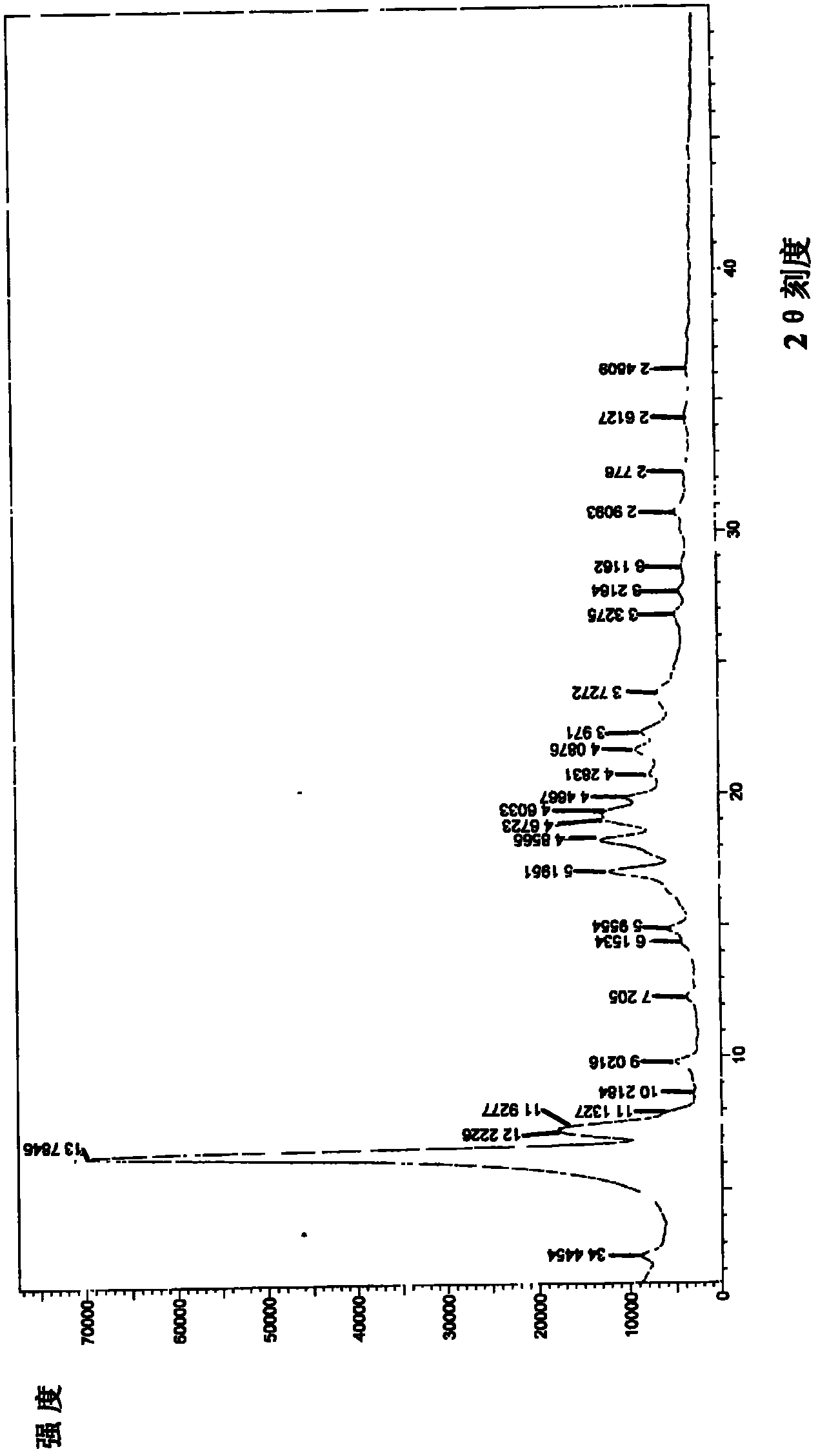
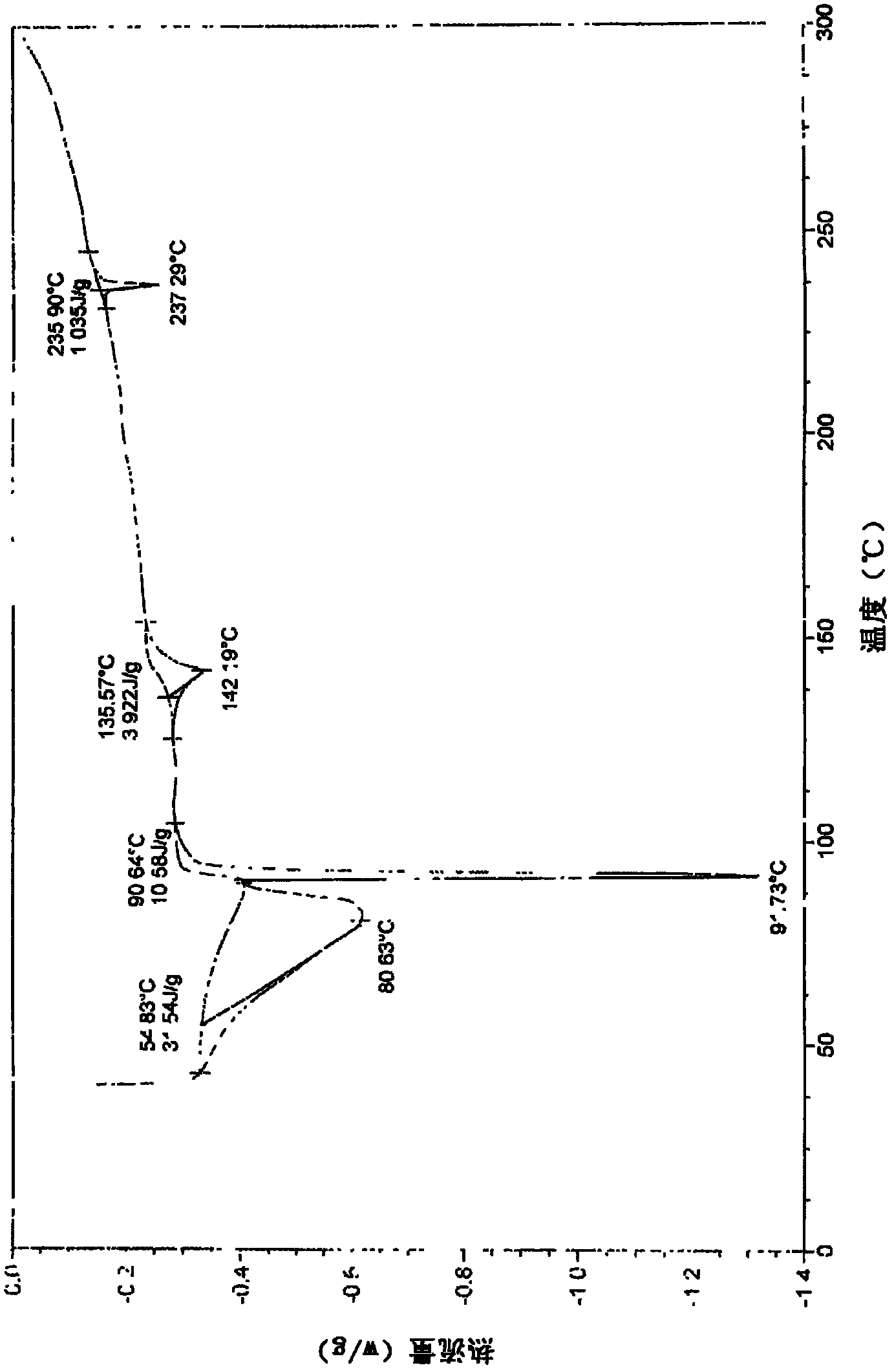
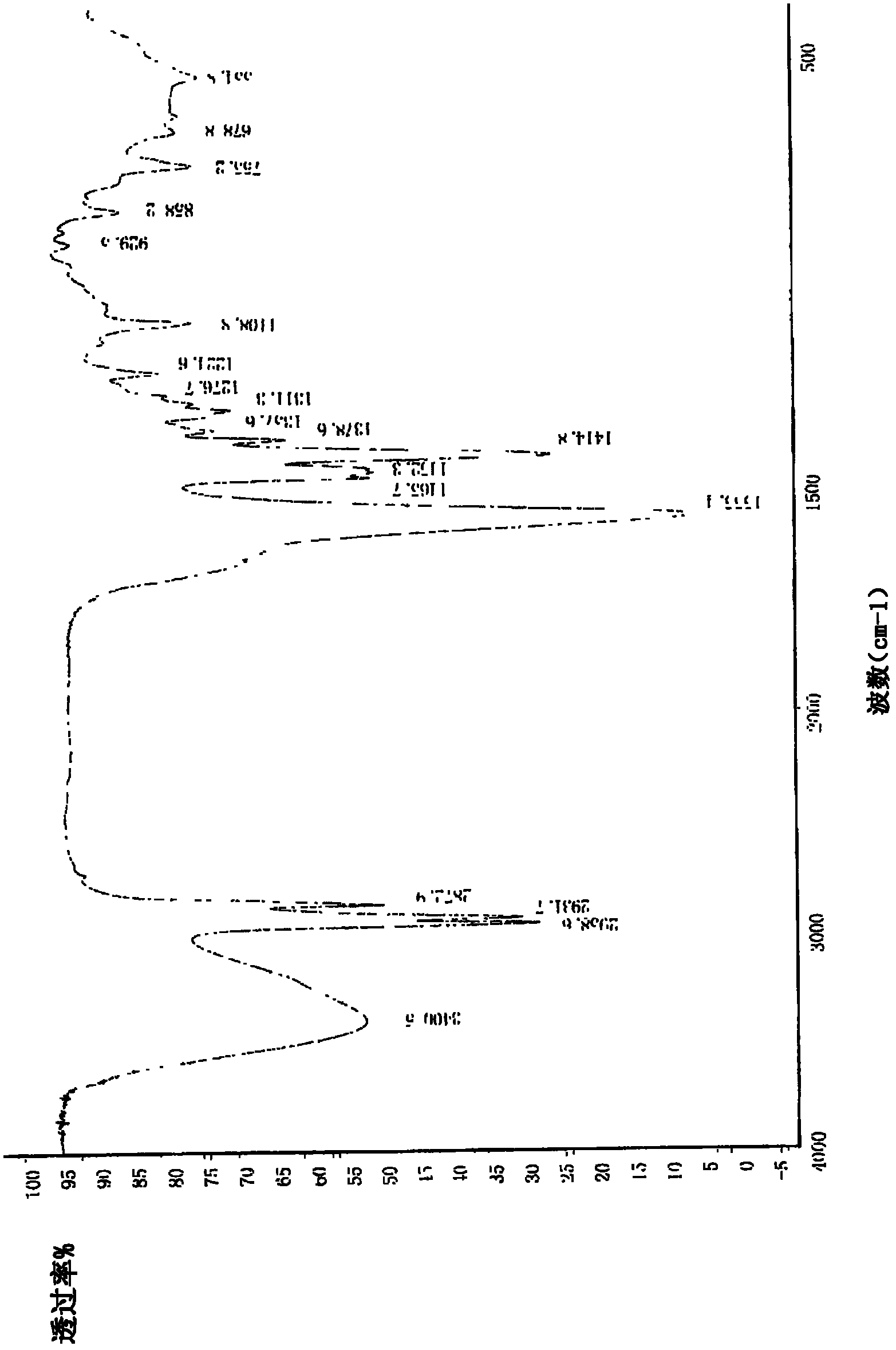
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of sodium valproate crystal form III of the present invention
[0035] (1) Dosing: Take 3 kg of sodium valproate (commercially available), dissolve it in 4 / 5 prescription water, stir and dissolve, add 0.1% (w / v) activated carbon and stir for 30 minutes, use 1 μm titanium Filtrate with a stick filter until clarified to obtain the filtrate; add water for injection to the prescription amount of sodium valproate concentration of 112 mg / ml, and adjust the pH to 7.9 with dilute hydrochloric acid. Filter with a 0.22 μm microporous membrane. Fill in 10ml vials (3.5ml per bottle).
[0036] (2) Freeze-drying process: the system automatically runs according to the freeze-drying curve of sodium valproate for injection (freeze-freeze preservation-primary drying-secondary drying). The water-catching condenser of the freeze dryer cools down to below -50°C, and the shelf temperature of the product is quickly frozen to -40°C after the product is in the drying box...
Embodiment 2
[0051] Example 2 Preparation of sodium valproate crystal form III of the present invention
[0052] (1) Dosing: Take 3 kg of sodium valproate, dissolve it in 4 / 5 prescription water, stir and dissolve, add 0.1% (w / v) activated carbon and stir for 30 minutes, then use a 1 μm titanium rod filter to filter to Clarify to obtain a filtrate; add water for injection until the concentration of sodium valproate in the prescribed amount is 133 mg / ml, and adjust the pH to 7.9 with dilute hydrochloric acid. Filter with a 0.22 μm microporous membrane. Fill in 7ml vials (3.0ml per bottle).
[0053] (2) Freeze-drying process: the system automatically runs according to the freeze-drying curve of sodium valproate for injection (freeze-freeze preservation-primary drying-secondary drying). The water-catching condenser of the freeze dryer cools down to below -50°C, and the shelf temperature of the product is quickly frozen to -40°C after the product is in the drying box of the freeze dryer, and ...
Embodiment 3
[0060] Example 3 Preparation of sodium valproate crystal form III of the present invention
[0061] (1) Dosing: Take 3 kg of sodium valproate, dissolve it in 4 / 5 prescription water, stir and dissolve, add 0.1% (w / v) activated carbon and stir for 30 minutes, then use a 1 μm titanium rod filter to filter to Clarify to obtain a filtrate; add water for injection until the concentration of sodium valproate in the prescribed amount is 160 mg / ml, and adjust the pH to 8.0 with dilute hydrochloric acid. Filter with a 0.22 μm microporous membrane. Fill in 7ml vials (2.5ml per bottle).
[0062] (2) Freeze-drying process: the system automatically runs according to the freeze-drying curve of sodium valproate for injection (freeze-freeze preservation-primary drying-secondary drying). The water-catching condenser of the freeze dryer cools down to below -50°C, and the shelf temperature of the product is quickly frozen to -40°C after the product is in the drying box of the freeze dryer, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com