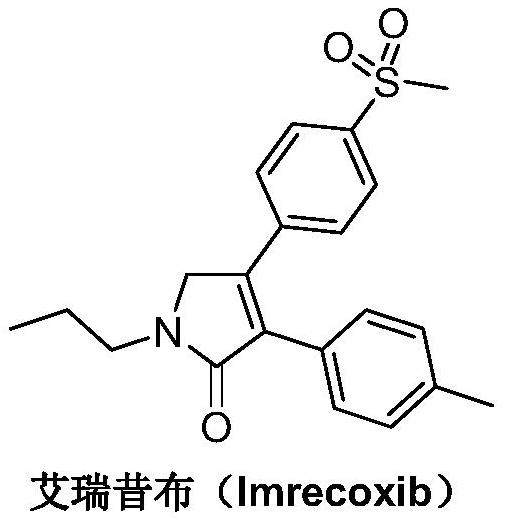
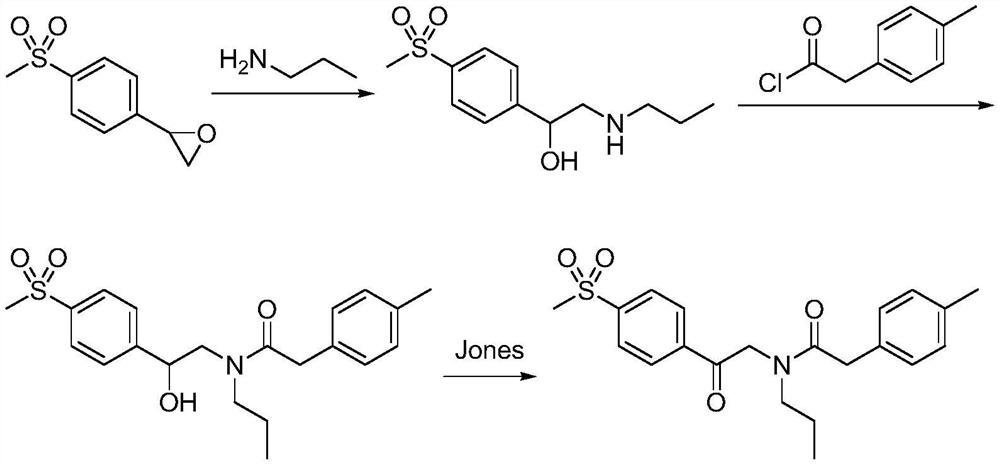
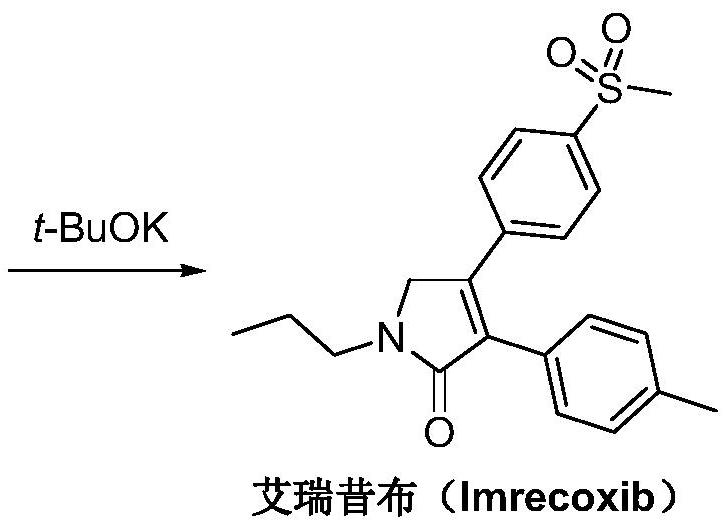
A kind of synthetic method of Erecoxib
A synthesis method and p-methylphenyl technology, applied in the field of erecoxib synthesis, can solve the problems of many impurities and by-products, unfavorable post-processing and purification, and difficulty in meeting the quality requirements of raw materials, and achieve cost reduction, The effect of less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A) Synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid methyl ester:
[0041] (E)-1-p-methylsulfonylphenyl-1-nitro-2-p-tolylethylene (21.0g) was dissolved in isopropanol (300mL), sodium hydroxide (4.0g) was added, stirred and ice-bathed Cool to 5-10°C, add a solution of methyl isocyanoacetate (7.2g) in isopropanol (12mL) dropwise, rise to 30°C and react for 18h until the reaction is complete. After post-treatment, the obtained crude product is recrystallized with ethanol, To obtain methyl 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylate, 23.2 g of off-white solid, yield 95%.
[0042] B) Synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carbaldehyde:
[0043]3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid methyl ester (23.0g) was dissolved in dichloromethane (350mL), cooled to -78°C, and bis(2-methyl Oxyethoxy) sodium aluminum hydride (13.8g) in n-hexane solution, heat preservation reaction for ...
Embodiment 2
[0049] A) synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid ethyl ester:
[0050] (E)-1-p-methylsulfonylphenyl-1-nitro-2-p-tolylethylene (12.0g) was dissolved in tetrahydrofuran (180mL), potassium carbonate (9.4g) was added, stirred and cooled to 5 ~10°C, add a solution of ethyl isocyanoacetate (5.6g) in tetrahydrofuran (10mL) dropwise, rise to 35°C and react for 12h until the reaction is complete. Ethylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylate, off-white solid 13.5g, yield 93%.
[0051] B) Synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carbaldehyde:
[0052] 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid ethyl ester (13.0g) was dissolved in toluene (180mL), cooled to -78°C, diisobutylaluminum hydride ( 6.3 g) of n-hexane solution, keep it warm for 4 hours until the reaction is complete, add dilute hydrochloric acid dropwise at low temperature to quench the reaction solution, rise to room temperatu...
Embodiment 3
[0058] A) Synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid methyl ester:
[0059] (E)-1-p-methylsulfonylphenyl-1-nitro-2-p-tolylethylene (5.6g) was dissolved in methanol (100mL), and 1,8-diazabicyclo[5.4.0]deca was added One-carb-7-ene (5.4g), stirred and cooled in an ice bath to 5-10°C, added dropwise a solution of methyl isocyanoacetate (2.6g) in methanol (6mL), raised to 20°C and reacted for 24h until the reaction was complete , after post-treatment, the obtained crude product was recrystallized from ethanol to obtain 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid methyl ester, 6.3 g of off-white solid, yield 96%.
[0060] B) Synthesis of 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carbaldehyde:
[0061] 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylic acid methyl ester (6.2g) was dissolved in chloroform (100mL), cooled to -78°C, diisobutylaluminum hydride ( 3.6 g) of n-hexane solution, heat preservati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com