Method for synthesizing cinacalcet
A technology of cinacalcet and compounds, applied in the field of pharmaceutical chemical synthesis, can solve the problems of high synthesis cost, residue, complicated operation, etc., and achieve the effect of simple synthesis method, avoiding residue, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Add 0.005mmol tris(pentafluorophenyl)boron to a 10mL Schlenk reaction tube (Beijing Shinwell Glass Instrument Co., Ltd., F891410 reaction tube, capacity 10mL, ground 14 / 20), replace the air in the tube with argon, and then Under an argon atmosphere, add 1.5 mL of n-butyl ether and 2.0 mmol of phenylsilane and stir (use IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). Then 0.5 mmol of (R)-1-(1-naphthyl)ethylamine and 1.0 mmol of 3-(3-trifluoromethylphenyl)propionic acid were added. After heating at 120° C. for 10 h, it was cooled to room temperature. Quenched with sodium hydroxide solution (3M; 3mL), added ethyl acetate (3mL), stirred at room temperature for 3h, extracted with ethyl acetate (2mL x 3), dried the organic phase with anhydrous sodium sulfate, filtered, and the organic phase Concentrate through a rotary evaporator (Buchi Co., Ltd., Switzerland, BUCHI rotary evaporator R-3), and then pass through a chromatography column (Beijing Xinweier Glass I...
Embodiment 2
[0060] The same procedure as Example 1 was followed, except that the reaction mixture was heated at 120° C. for 18 h and then cooled to room temperature. After the same post-treatment, a white crystal product was isolated with a yield of 81%.
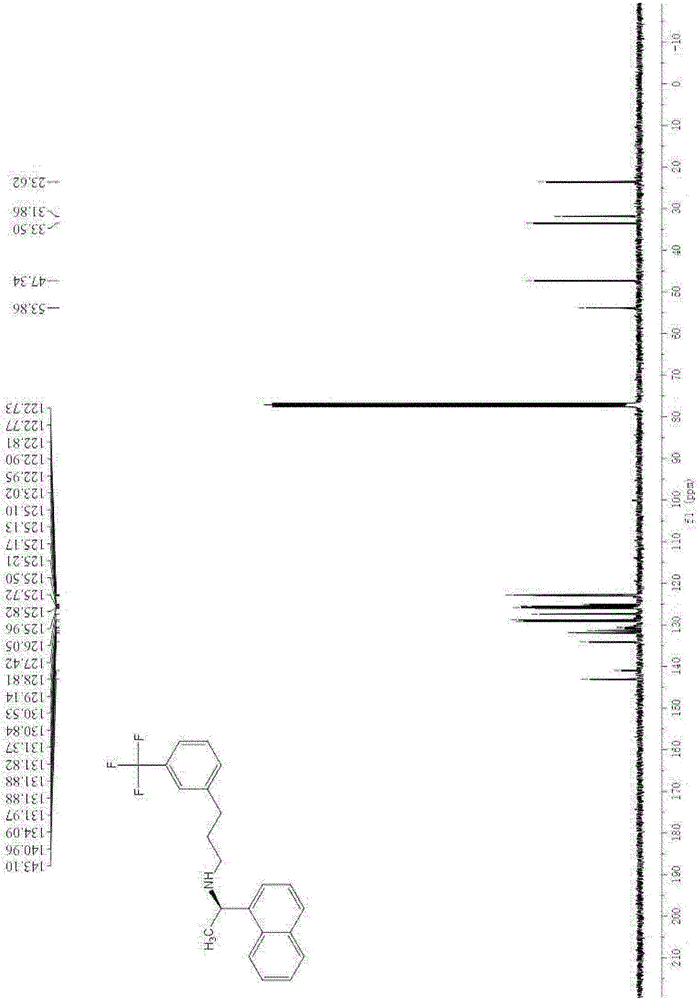
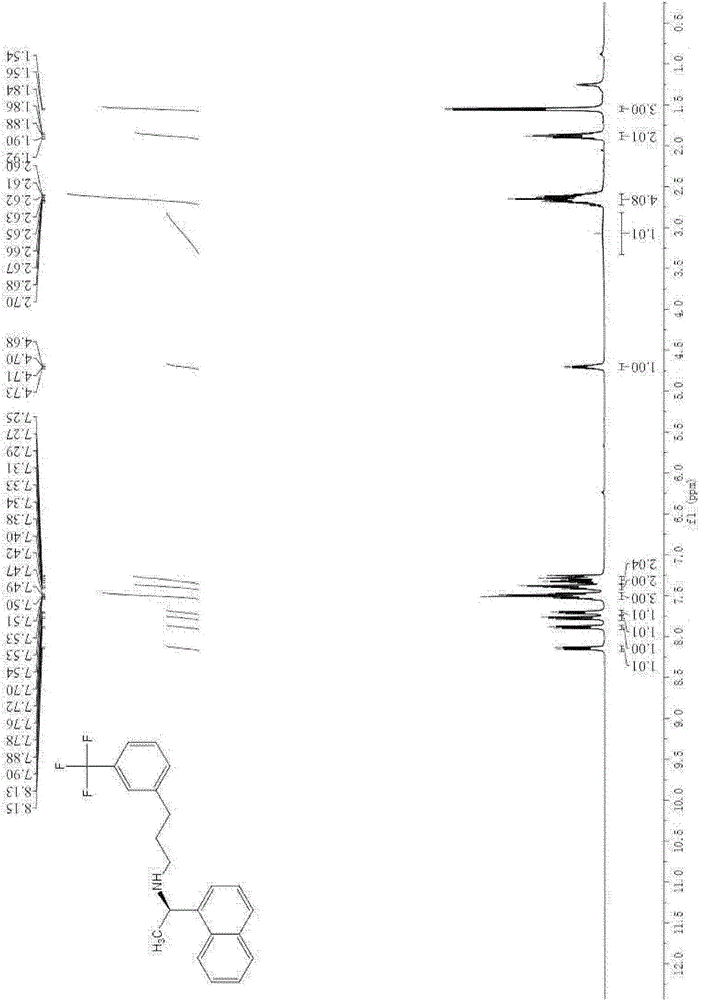
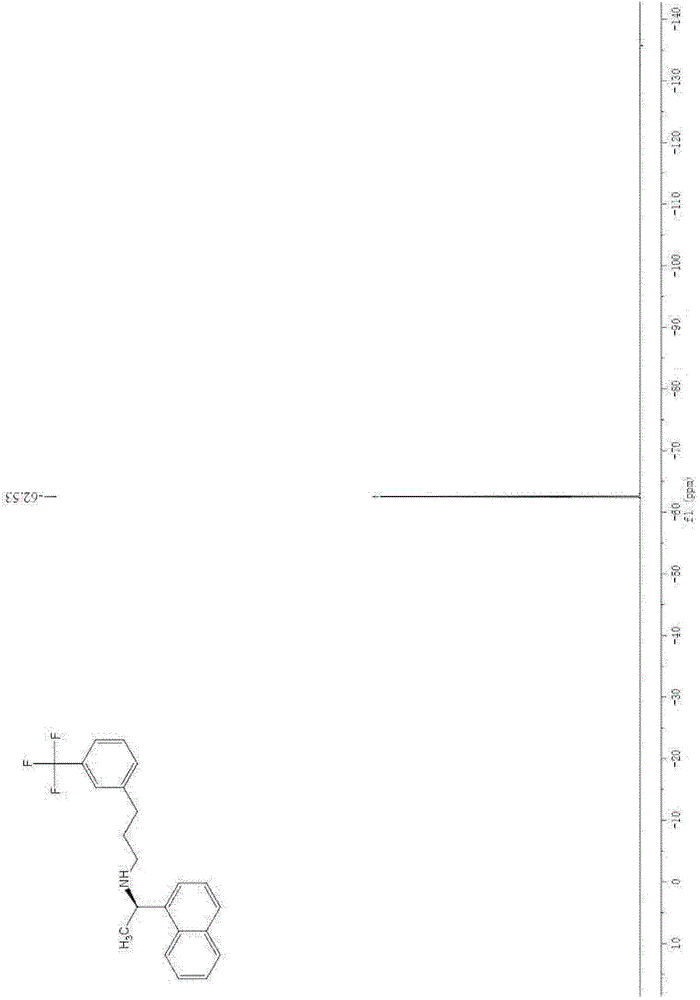
[0061] After proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum and fluorine nuclear magnetic resonance spectrum analysis, it was confirmed that the resulting product was N-((1R)-1-(1-naphthyl)ethyl)-3-(3-(trifluoromethyl) ) phenyl) propan-1-amine, namely cinacalcet.
Embodiment 3
[0063] The same procedure as Example 1 was followed, except that the reaction mixture was heated at 120° C. for 20 h and then cooled to room temperature. After the same post-treatment, a white crystal product was isolated with a yield of 88%.
[0064] After proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum and fluorine nuclear magnetic resonance spectrum analysis, it was confirmed that the resulting product was N-((1R)-1-(1-naphthyl)ethyl)-3-(3-(trifluoromethyl) ) phenyl) propan-1-amine, namely cinacalcet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com