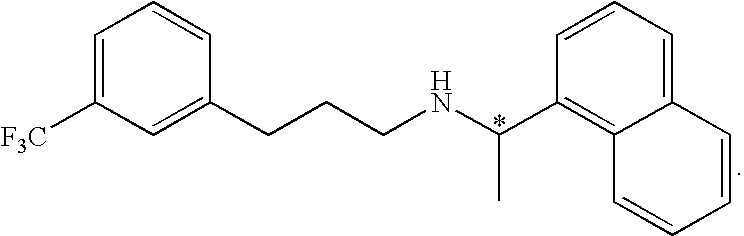
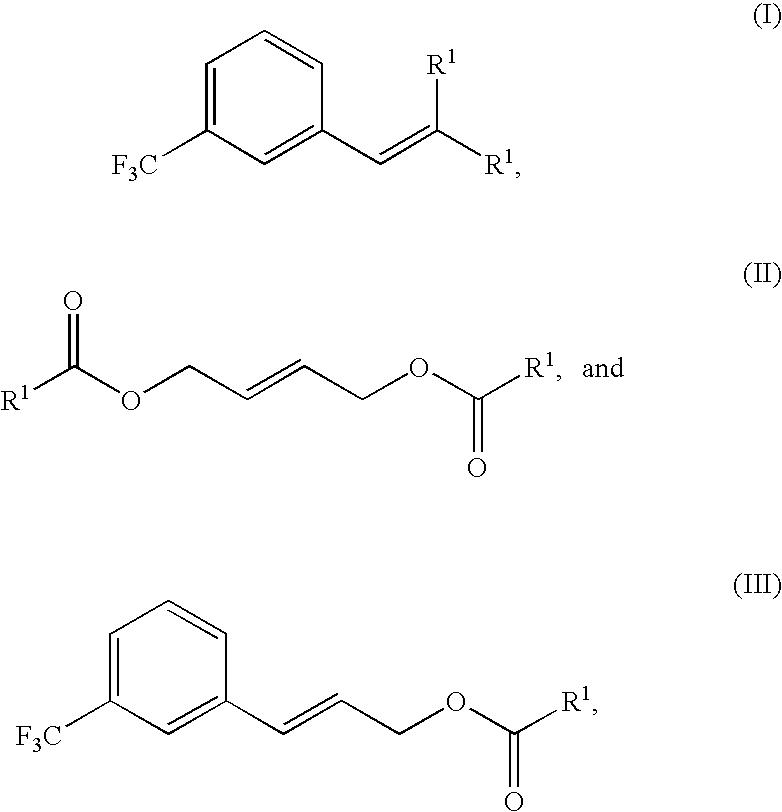
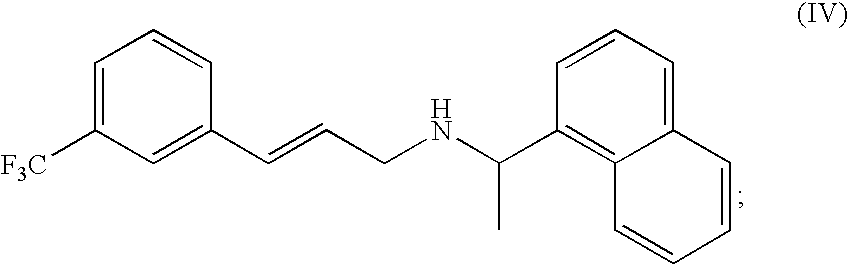
Methods of synthesizing cinacalcet and salts thereof
a technology of cinacalcet and calcimimetic agent, which is applied in the preparation of amino compounds, drug compositions, metabolic disorders, etc., can solve the problems of cardiovascular morbidity, abnormal calcium and phosphorus levels, and serious consequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Allylic Amination
[0109]
[0110](R)-(1-Naphthyl)ethylamine (257 mg, 1.5 mmol) and 3-trifluoromethyl cinnamyl acetate (244 mg, 1 mmol) were charged to a 15 mL flask. Tetrahydrofuran (THF, 2 mL) and tetrakis(triphenylphosphine)palladium (58 mg, 5 mol %) were added, and the flask was purged with nitrogen. The reaction mixture was stirred at room temperature for 16 h. The solvents were evaporated under vacuum and the residue was dissolved in dichloromethane (10 mL). The organic phase was washed with saturated sodium bicarbonate (5 mL) and the aqueous phase was back extracted with dichloromethane (5 mL). The organic layers were dried over magnesium sulfate and evaporated to dryness. Chromatography on silica gel using dichloromethane:methanol (50:1) as eluent afforded the desired (R)-(1-naphthalen-1-yl-ethyl)-[(E)-3-(3-trifluoromethyl-phenyl)-allyl}-amine (294 mg, 98.1% HPLC purity at 254 nm, 83% uncorrected yield). The product was contaminated by some triphenylphosphine. The dialkylated pro...
example 2
[0116]In an alternative embodiment the present invention provides a methodology for synthesizing cinacalcet by a reaction between the appropriate amine and a pre-activated carboxylic acid.
[0117]The reaction was initiated by the addition of trans-3-trifluromethyl cinnamoyl chloride (1.0 equiv.; 21.3 mmol; 5 g) to a stirred solution of (R)-(+)-1-(1-napthyl)ethylamine (1.1 equiv.; 23.4 mmol; 4 g) and triethylamine (1.1 equiv.; 23.4 mmol; 3.26 mL) in toluene (100 mL) in an inert atmosphere of nitrogen. After stirring for 2 hours at room temperature, water (100 mL) was added to precipitate the crude product as a white solid. The solid was filtered and dried in a vacuum oven over night at 55° C. Yield: 82%; 6.49 g. The identity of (E)-N-((R)-1-Naphthalen-1-yl-ethyl)-3-(3-trifluoromethyl-phenyl)-acrylamide was confirmed by 1H NMR (400 MHz, CDCl3) and high resolution mass spectrometry. δ ppm 1.76 (d, 3H) 5.85 (d, 1H) 6.08 (quin, 1H) 6.39 (d, 1H) 7.43-7.73 (m, 9H) 7.80-7.91 (...
example 3
Metal Catalyzed Cross-Coupling Reaction
[0126]Cinacalcet can be synthesized via a metal catalyzed coupling reaction between an appropriately substituted aryl halide and N-((R)-1-Naphthalen-1-yl-ethyl)-acrylamide. The acrylamide was synthesized by adding acryloyl chloride (1.2 equiv.; 17.5 mmol; 1.58 g) via syringe to an ice cold solution of (R)-(+)-1-(1-napthyl)ethylamine (2.50 g, 14.6 mmol, 1.0 eq.), and triethylamine (1.2 equiv.; 17.5 mmol; 1.77 g) in toluene (50 ml). After the addition of acryloyl chloride is complete, the reaction mixture was allowed to warm to room temperature. The reaction was quenched after stirring for an hour at room temperature by adding water (50 mL) to the reaction mixture. The crude product which precipitates out was filtered and dried in vacuo. Crude yield 79%; 3.1 g. The identity of the product was confirmed by 1H NMR and LC / MS. (400 MHz, CDCl3) δ ppm 1.72 (d, 3H) 5.63 (dd, 1H) 5.68-5.78 (m, 1H) 5.96-6.08 (m, 2H) 6.31 (dd, 1H) 7.42-7.59 (m, 4H) 7.77-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com