Oral solid rapid release preparation of cinacalcet hydrochloride
A technology for cinacalcet hydrochloride and immediate-release preparations, applied in the field of medicine, can solve the problems of complicated operation, inability to control the dissolution rate and amount of cinacalcet hydrochloride well, unfavorable health and the like, and achieves simple preparation process, Improve the dissolution rate and dissolution amount, and the effect of controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the cinacalcet hydrochloride tablet of the present invention adopts a wet granulation process, that is, through micronization, sieving, mixing, granulation, drying, granulation, tabletting and other technical processes.
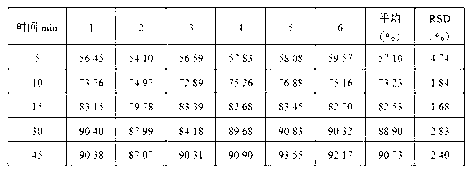
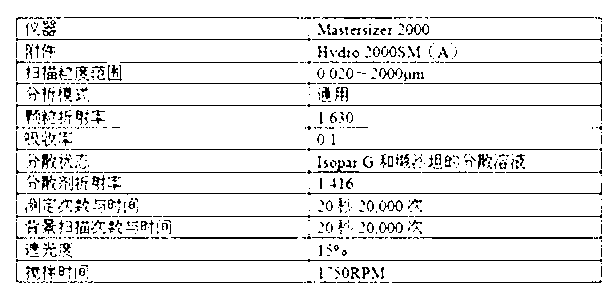
[0028] Particle size distribution determination method:
[0029] a) method parameters
[0030]
[0031] b) Determination process
[0032] The sample to be analyzed was prepared by weighing 60 mg of cinacalcet hydrochloride into 20 mL of sample dispersant, which was previously prepared by diluting 1.5 g of soybean lecithin to 200 mL with Isopar G. The suspension was added dropwise to a background-corrected measuring cell previously filled with a dispersant (Isopar G) until the refractive index reached approximately 15%. After the measurement is completed, the sample cell is emptied and rinsed, the suspension medium is refilled, and the sampling procedure is repeated here. For characterization, specifically list D 10 、D ...
Embodiment 1
[0048] Example 1 Preparation of oral solid immediate-release formulation of cinacalcet hydrochloride
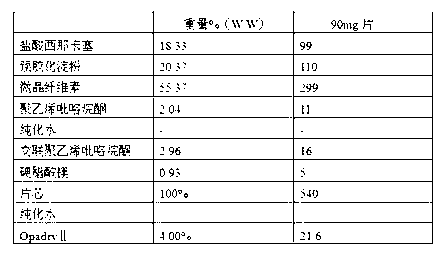
[0049] Preparation of raw material composition containing 90 mg (calculated as cinacalcet hydrochloride free base) active ingredient of specified particle size distribution
[0050]
[0051] The preparation of the oral solid immediate-release preparation of cinacalcet hydrochloride comprises the following steps:
[0052] (1) Micronize cinacalcet hydrochloride, weigh it, and sieve it to obtain particles with an average particle size of 5-15 μm, prepare a certain concentration of adhesive solution, and then mix cinacalcet hydrochloride with filler and disintegrate The agent is mixed evenly;
[0053] (2) Add the binder into the uniformly mixed powder of the main ingredient and excipients, granulate, granulate, and dry at a certain temperature until the moisture meets the requirements, that is, dry granules are obtained;
[0054] (3) Then add a disintegrating agent and a lub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com