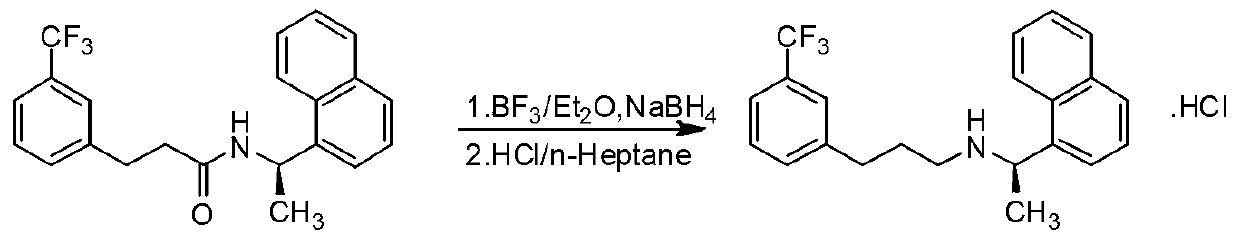Preparation method of cinacalcet
A technology of cinacalcet and solvent, which is applied in the field of cinacalcet preparation, can solve problems such as inconvenience, and achieve the effects of low preparation cost, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
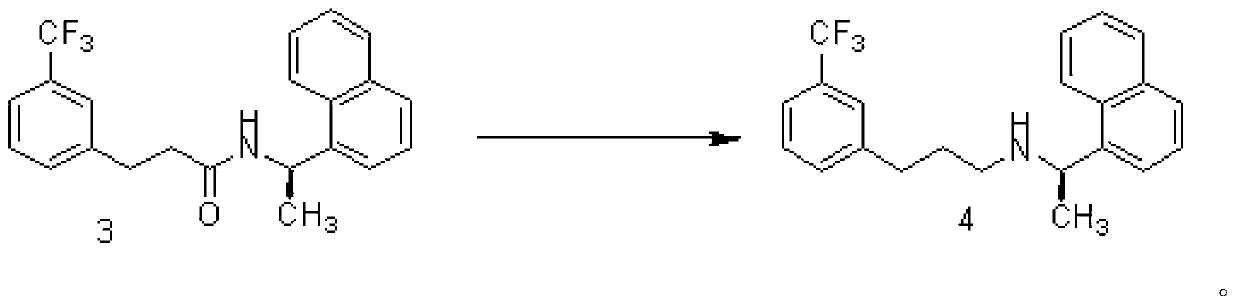
[0040] Synthesis of N-[1-(R)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-propionamide (4)
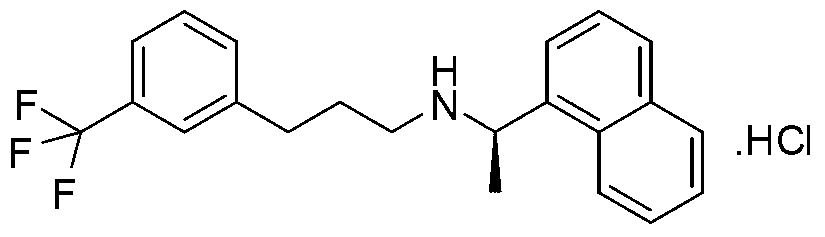
[0041] Add 250ml THF, ZnCl 2 (108.7g, 0.8mol), KBH 4 (86.4g, 1.6mol), stirred at 25°C for 3 hours. Add compound (3) (74.2 g, 0.2 mol) and 100 ml of toluene to the reaction liquid, heat, evaporate part of the solvent until the internal temperature rises to 95 ° C, and stir for 5 h. The reaction solution was cooled to room temperature 25°C, poured into 250ml of hydrochloric acid with a volume ratio of 1%, and filtered to remove solid salts. The mother liquor was extracted with ethyl acetate (100ml×3), separated, and the water phase was treated with 20% sodium hydroxide by weight. The aqueous solution was basified to PH=11~12, then extracted with ethyl acetate, the organic phase extracts were combined, and washed with anhydrous Na 2 SO 4 Dry, filter, concentrate the mother liquor, obtain off-white solid target compound (4), dry weighing 67.8g, yield 95%
Embodiment 2
[0043] Synthesis of N-[1-(R)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-propionamide (4)
[0044] Add 250ml THF, ZnCl 2 (108.7g, 0.8mol), NaBH 4 (60.8g, 1.6mol), stirred at 25°C for 3 hours. Compound (3) (74.2g, 0.2mol) and 50ml of toluene were added to the reaction liquid, heated slowly, part of the solvent was evaporated to raise the internal temperature to 98°C, and the reaction was stirred for 5h. The reaction solution was cooled to room temperature 25°C, poured into 250ml of hydrochloric acid with a volume ratio of 1%, and filtered to remove solid salts. The mother liquor was extracted with ethyl acetate (100ml×3), separated, and the water phase was treated with 20% sodium hydroxide by weight. The aqueous solution was basified to PH=11~12, then extracted with ethyl acetate, the organic phase extracts were combined, and washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the mother liquor, the target compound (4) was obtained as an off-wh...
Embodiment 3
[0046] Synthesis of N-[1-(R)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-propionamide (4)
[0047] Add 250ml THF, MgCl 2 (75.1g, 0.8mol), KBH 4(86.4g, 1.6mol), stirred at 25°C for 3 hours. Compound (3) (74.2g, 0.2mol) and 50ml of toluene were added to the reaction liquid, heated slowly, part of the solvent was evaporated to raise the internal temperature to 100°C, and the reaction was stirred for 5h. The reaction solution was cooled to room temperature 25°C, poured into 250ml of hydrochloric acid with a volume ratio of 1%, and filtered to remove solid salts. The mother liquor was extracted with ethyl acetate (100ml×3), separated, and the water phase was treated with 20% sodium hydroxide by weight. The aqueous solution was basified to PH=11~12, then extracted with ethyl acetate, the organic phase extracts were combined, and washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the mother liquor, the target compound (4) was obtained as an off-whit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com