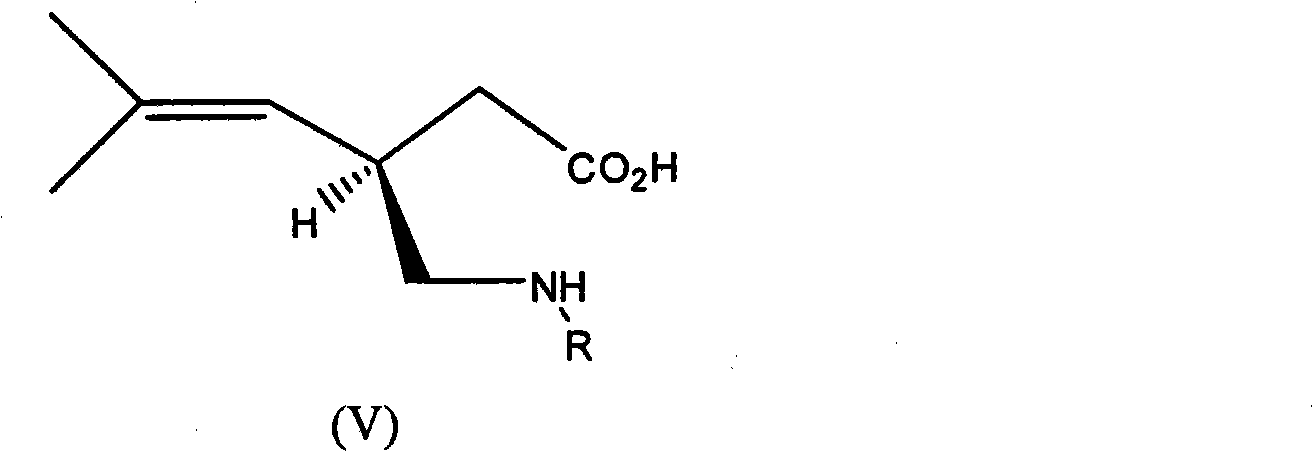
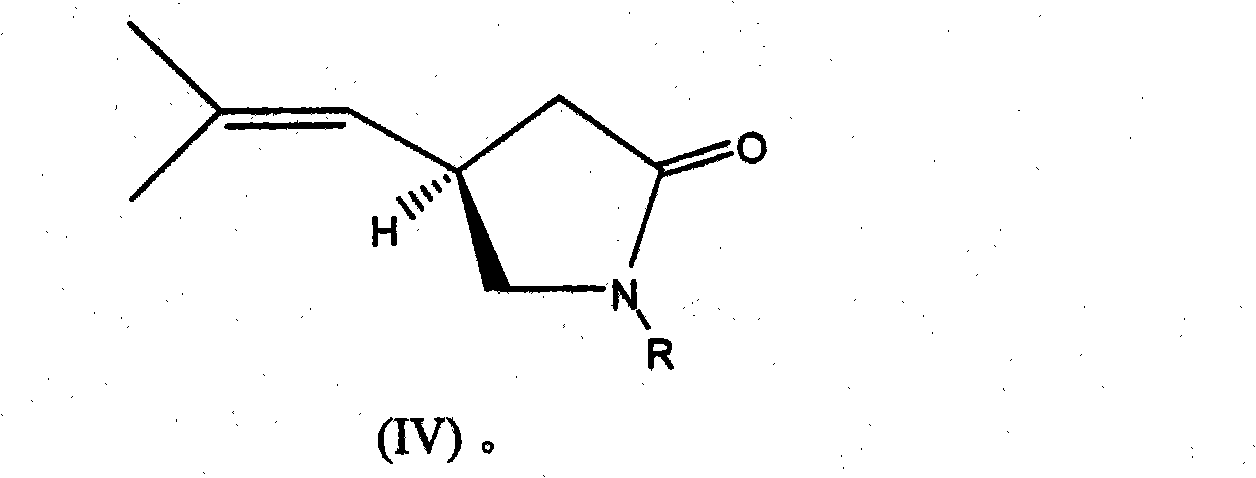
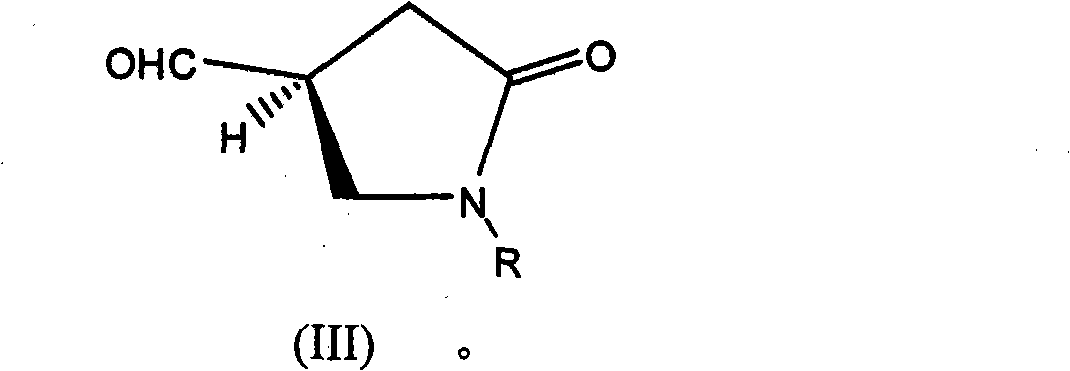
Process for the enantioselective preparation of pregabalin
An enantioselective, pregabalin technology, applied in the field of enantioselective preparation-pregabalin or its salts, can solve problems such as high cost of catalyst systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] 1.1 Synthesis of (R)-2,2-dimethyl-1,3-dioxolane-4-aldehyde
[0092]
[0093] NaIO 4 (16g, 74.8mmol) added (1S,2S)-1,2-bis((R)-2,2-dimethyl-1,3-dioxolan-4-yl)ethane-1,2 -diol (20g, 76.2mmol) in 9:1 THF-H 2 In a stirred solution in O (280 mL), the resulting mixture was stirred for 4 h. The resulting precipitate was filtered off and most of the THF was evaporated under reduced pressure. Water (20 mL) was then added, and the aqueous solution was extracted with dichloromethane (6 x 50 mL). Combined organic extracts in MgSO 4 ((R)-2,2-Dimethyl-1,3-dioxolane-4-carbaldehyde (16.8 g, 85% yield), which was used without further purification In the next steps.
[0094] · 1 H-NMR (250MHz, CDCl 3 )
[0095] 1.4(s, 3H), 1.5(s, 3H), 4.1(m, 2H), 4.4(m, 1H), 9.7(d, 3 J H-H = 2Hz, 1H).
[0096] IR (film): 3417, 2985, 1735, 1372, 1064cm -1 .
[0097] ·Appearance: Colorless oil.
[0098] 1.2 Synthesis of (E)-3-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)acrylate
[0099]
[01...
Embodiment 2
[0165] 2.1 Synthesis of (S)-1-benzyl-4-((S)-2,2-dimethyl-1,3-dioxolane-4-yl)pyrrolidin 2-one
[0166]
[0167] Sodium hydride (60%, 0.24 g, 6.0 mmol) in paraffin oil was added to (S)-4-((S)-2,2-dimethyl-1,3-dioxolane on an ice bath -4-yl)pyrrolidin-2-one (1.0 g, 5.4 mmol) in a stirred solution of anhydrous THF (75 mL). The mixture was stirred at 0°C for 30 min, and benzyl bromide (0.7 mL, 5.7 mmol) was added dropwise. The reaction mixture was allowed to warm to room temperature and stirred for another 3 h. The solvent was evaporated under vacuum, and the residue was poured into EtOAc (100 mL), washed several times with water. Organic phase in MgSO 4 Dry and remove the solvent. The residue (0.8g) was in Chromatography on silica gel with EtOAc-hexane (2:3) as eluent afforded pure (S)-1-benzyl 4-((S)-2,2-dimethyl-1,3- Dioxolan-4-yl)pyrrolidin-2-one (0.9 g, 60% yield).
[0168] · 1 H-NMR (250MHz, CDCl 3 )
[0169] 1.3(s, 3H), 1.4(s, 3H), 2.2(m, 1H), 2.5(m, 2H), 3.3(m...
Embodiment 3
[0219] 3.1 (S)-tert-butyl 4-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-2-oxopyrrolidine-1-carboxylate synthesis
[0220]
[0221] To (S)-4-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)pyrrolidin-2-one (500 mg, 2.7 mmol) in dichloromethane ( To a stirred solution in 25 mL), triethylamine (0.4 mL, 2.9 mmol), 4-dimethylaminopyridine (330 mg, 2.7 mmol) and Boc anhydride (1.2 mL, 5.4 mmol) were added successively. The light protected mixture was stirred at room temperature for 12 h. The solvent was evaporated under vacuum and the residue was Chromatography on silica gel using EtOAc-hexane (1:2) as eluent gave pure (S)-tert-butyl 4-((S)-2,2-dimethyl-1,3- Dioxolane4-yl) 2-oxopyrrolidine-1-carboxylate (770 mg, 100% yield), could be crystallized in EtOAc / pentane.
[0222] Characterization:
[0223] · 1 H-NMR (250MHz, CDCl 3 )
[0224] 1.4(s, 3H), 1.5(s, 3H), 1.6(s, 9H), 2.3(m, 1H), 2.4(m, 1H), 2.6(m, 1H), 3.7(m, 2H), 3.9 (dd, 3 J H-H = 6Hz, 3 J H-H = 10.5 Hz, 1H), 4.1 (m, 2H).
[02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com