Method of synthesizing GHK acetate at low cost
An acetate, low-cost technology, applied in the preparation method of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of increased by-products, easy removal, cumbersome operation, etc., to reduce production costs and avoid cumbersome Sexuality, avoiding the effect of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
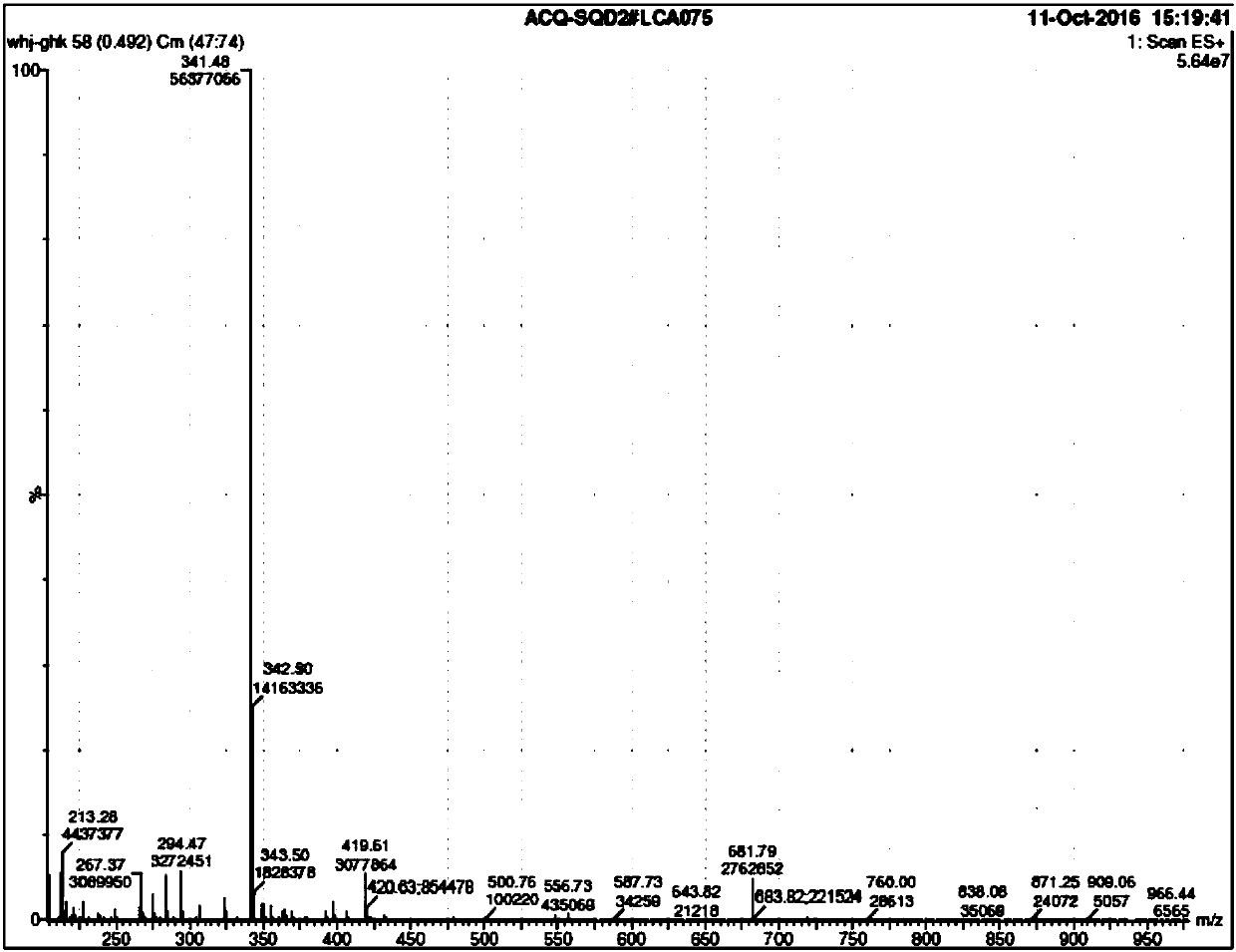
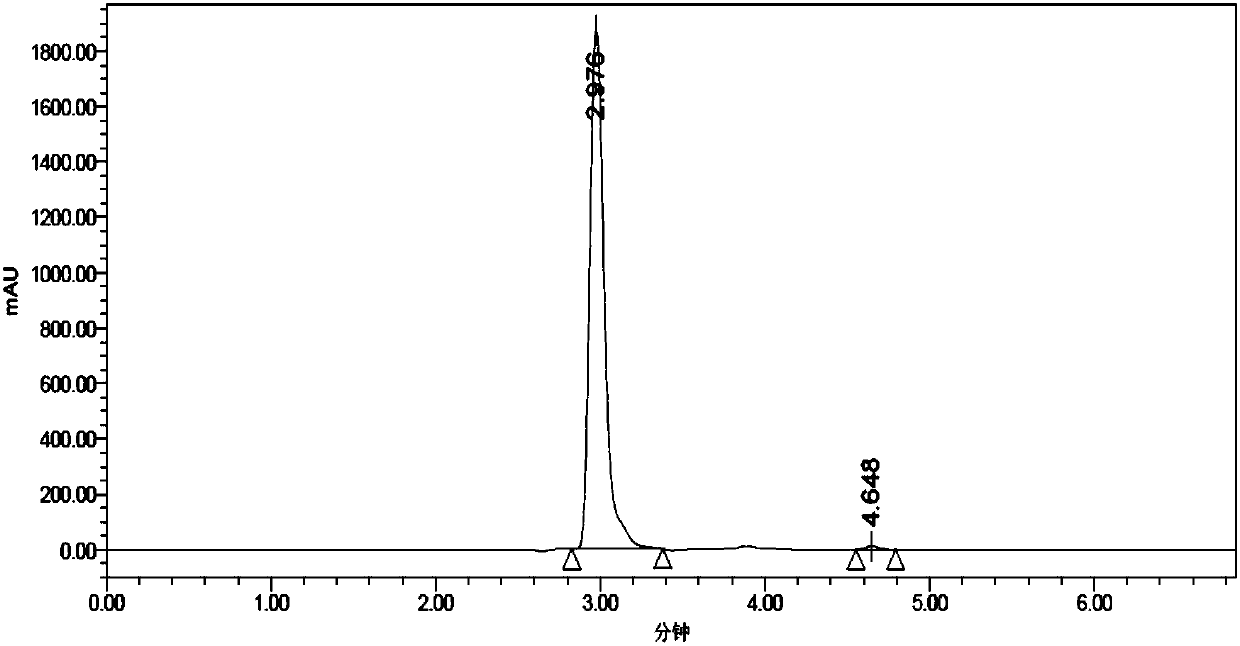
Image
Examples
Embodiment 1
[0030] 1. Synthesis of Trt-Gly-OSu
[0031] Add 31.7g (0.1mol) Trt-Gly-OH and 300mL tetrahydrofuran into the reaction flask, stir at room temperature to dissolve and clear, add 13.8g (0.12mol) N-hydroxysuccinimide, stir to dissolve and clear, then add 30.9g ( 0.15mol) N,N'-dicyclohexylcarboimide, after stirring at room temperature for 30 minutes, a large amount of white solids precipitated, continued stirring for 3 hours, TLC showed that the reaction of the raw materials was complete, filtered, and the filtrate was concentrated under reduced pressure at 35°C , to obtain 41.4g Trt-Gly-OSu, yield 100%.
[0032] 2. Synthesis of Trt-Gly-His(Trt)-OH
[0033] Dissolve 41.4g (0.1mol) Trt-Gly-OSu in 300mL N,N-dimethylformamide, add 39.7g (0.1mol) His(Trt)-OH, 12.9g (0.1mol) N,N- Diisopropylethylamine, after stirring at room temperature for 1 hour, TLC showed that the raw materials were completely reacted. Pour the reaction solution into 1.5L water, adjust the pH to 3~4 with citric a...
Embodiment 2
[0042] 1. Synthesis of Trt-Gly-OSu
[0043] Add 95.1g (0.3mol) Trt-Gly-OH and 900mL tetrahydrofuran into the reaction flask, stir at room temperature to dissolve and clear, add 38g (0.33mol) N-hydroxysuccinimide, stir to dissolve and clear, then add 80.3g (0.39 mol) N,N'-dicyclohexylcarboimide, after stirring at room temperature for 30 minutes, a large amount of white solids precipitated, and continued stirring for 3 hours, TLC showed that the raw materials were completely reacted, filtered, and the filtrate was concentrated under reduced pressure at 35°C, 120.4 g of Trt-Gly-OSu were obtained with a yield of 97%.
[0044] 2. Synthesis of Trt-Gly-His(Trt)-OH
[0045] Dissolve 120.4 (0.29mol) Trt-Gly-OSu in 900mL N,N-dimethylformamide, add 127g (0.32mol) His(Trt)-OH, 41g (0.32mol) N,N-diisopropyl Ethylamine, after stirring at room temperature for 1 hour, TLC showed that the raw materials were completely reacted, the reaction solution was poured into 1.5L water, and the pH was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com