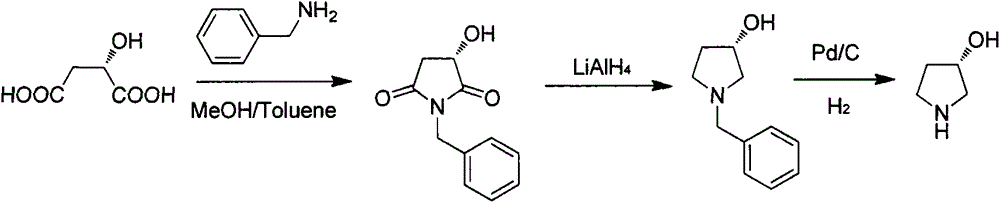
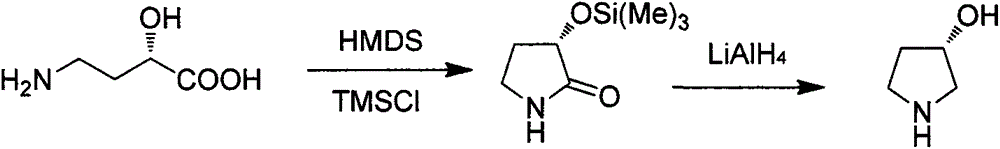
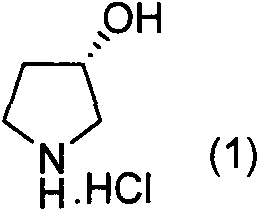
Preparation method of (S)-3-hydroxypyrrolidine hydrochloride
A hydroxypyrrolidine hydrochloride and hydroxypyrrolidine technology are applied in the field of preparation of -3-hydroxypyrrolidine hydrochloride, can solve problems such as inability to meet industrialized production, avoid column chromatography purification, reduce risk factor, Guaranteed effect of optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described below through the examples, but the examples do not limit the protection scope of the present invention.
[0027] 1. Preparation of (R)-1-tert-butoxycarbonyl-3-benzoyloxypyrrolidine (3)
[0028] Dissolve 50g (0.267mol) of (R)-1-N-tert-butoxycarbonyl-3-hydroxypyrrolidine, 42.4g (0.348mol) of benzoic acid, and 91g (0.348mol) of triphenylphosphine in 200ml of dry tetrahydrofuran, Under nitrogen protection, stir at -10°C. Slowly add 68.4ml (0.384mol) of diisopropyl azodicarboxylate dropwise, and control the internal temperature not to exceed -5°C. The dropwise addition time was about 30 minutes, and after the addition was completed, the temperature was slowly raised to room temperature, and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, 200ml of water was added to the residue, extracted with ethyl acetate (100ml×3), the organic phases were combined, washed with water (50ml×2) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com