Method for preparing and detecting intermediate and corresponding isomer of afatinib
An enantiomer and afatinib technology, which is applied in the field of content detection of key intermediates and their enantiomers, can solve the problems of different adsorption and desorption capabilities, and large differences in substituents of key intermediates. , to achieve the effect of ensuring precision and accuracy and ensuring optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
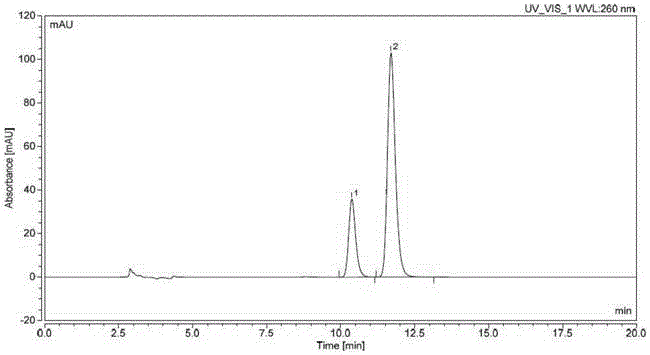
[0029] Chromatographic conditions: use cellulose-tris(3,5-dimethylphenylcarbamate) as filler (reference column: LuxChiralCellulose-1 (250*4.6mm, 5μm)), column temperature is 25°C, Using n-hexane-isopropanol-acetonitrile (750:200:50) as the mobile phase, the flow rate is 0.9ml per minute, the detection wavelength is 260nm, and the analysis time is 30 minutes.
[0030] Detection method: take N4-(3-chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazoline diamine compound (formula 2) 10mg, accurately weighed, put in a 20ml measuring bottle, add isopropanol to dissolve and dilute to the mark, shake well, and use it as the test solution. Take an appropriate amount of N4-(3-chloro-4-fluorophenyl)-7-[[(3R)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine compound (Formula 3) , add isopropanol to dissolve and quantitatively dilute to make a solution containing 2.5 μg per 1 ml, as the reference solution. Take 10 mg of the compound of formula 2 and 2 mg of the compound of ...
Embodiment 2
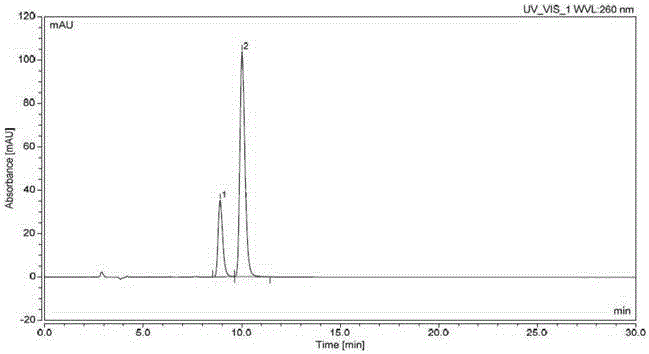
[0047] Chromatographic conditions: use cellulose-tris(3,5-dimethylphenylcarbamate) as filler (reference column: LuxChiralCellulose-1 (250*4.6mm, 5μm)), column temperature is 25°C, Using n-hexane-isopropanol-acetonitrile (700:250:50) as the mobile phase, the flow rate is 0.9ml per minute, the detection wavelength is 260nm, and the analysis time is 30 minutes.
[0048] Detection method: take N4-(3-chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazoline diamine compound (formula 2) 10mg, accurately weighed, put in a 20ml measuring bottle, add isopropanol to dissolve and dilute to the mark, shake well, and use it as the test solution. Take an appropriate amount of N4-(3-chloro-4-fluorophenyl)-7-[[(3R)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine compound (Formula 3) , add isopropanol to dissolve and quantitatively dilute to make a solution containing 2.5 μg per 1 ml, as the reference solution. Take 10 mg of the compound of formula 2 and 2 mg of the compound of ...
Embodiment 3
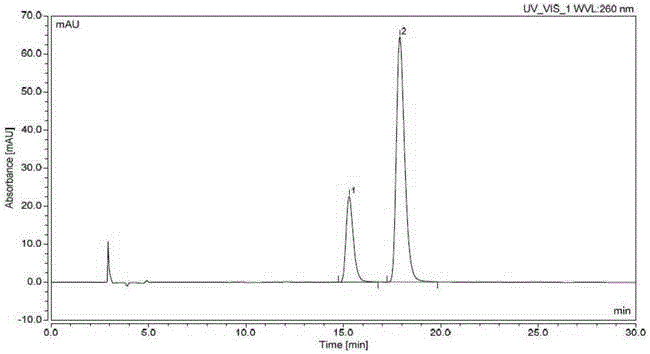
[0061] Chromatographic conditions: use cellulose-tris(3,5-dimethylphenylcarbamate) as filler (reference column: LuxChiral Cellulose-1 (250*4.6mm, 5μm)), column temperature is 25°C , using n-hexane-isopropanol-acetonitrile (800:150:50) as the mobile phase, the flow rate is 0.9ml per minute, the detection wavelength is 260nm, and the analysis time is 30 minutes.
[0062] Detection method: take N4-(3-chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazoline diamine compound (formula 2) 10mg, accurately weighed, put in a 20ml measuring bottle, add isopropanol to dissolve and dilute to the mark, shake well, and use it as the test solution. Take an appropriate amount of N4-(3-chloro-4-fluorophenyl)-7-[[(3R)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine compound (Formula 3) , add isopropanol to dissolve and quantitatively dilute to make a solution containing 2.5 μg per 1 ml, as the reference solution. Take 10 mg of the compound of formula 2 and 2 mg of the compound o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com