Patents
Literature
50 results about "Phenylcarbamates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenyl esters of carbamic acid or of N-substituted carbamic acids. Structures are similar to PHENYLUREA COMPOUNDS with a carbamate in place of the urea.
Phenylcarbamate compound and muscle relaxant containing the same
A novel phenylcarbamate compound and a pharmaceutical composition containing the same are provided. More specifically, a novel phenylcarbamate compound, a composition for muscle relaxation containing the phenylcarbamate compound as an active ingredient, and a method of muscle relaxation comprising administering a therapeutically effective amount of the phenylcarbamate compound, are provided.
Owner:BIO PHARM SOLUTIONS
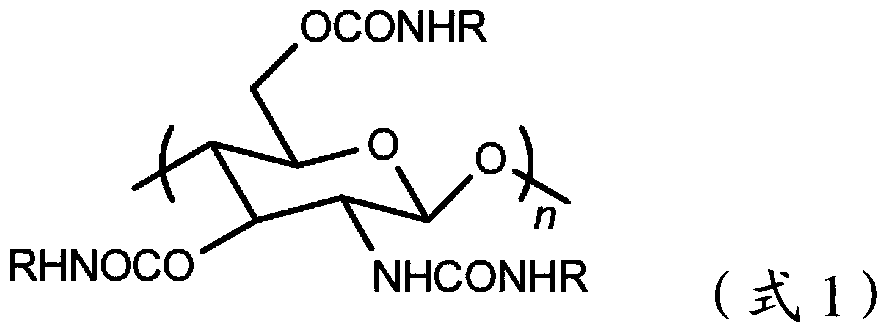
Chitosan carbanilate-carbamido derivative preparation method
ActiveCN104250312AImprove solubilitySmall structureOther chemical processesCarbamateChitosan phenylcarbamate
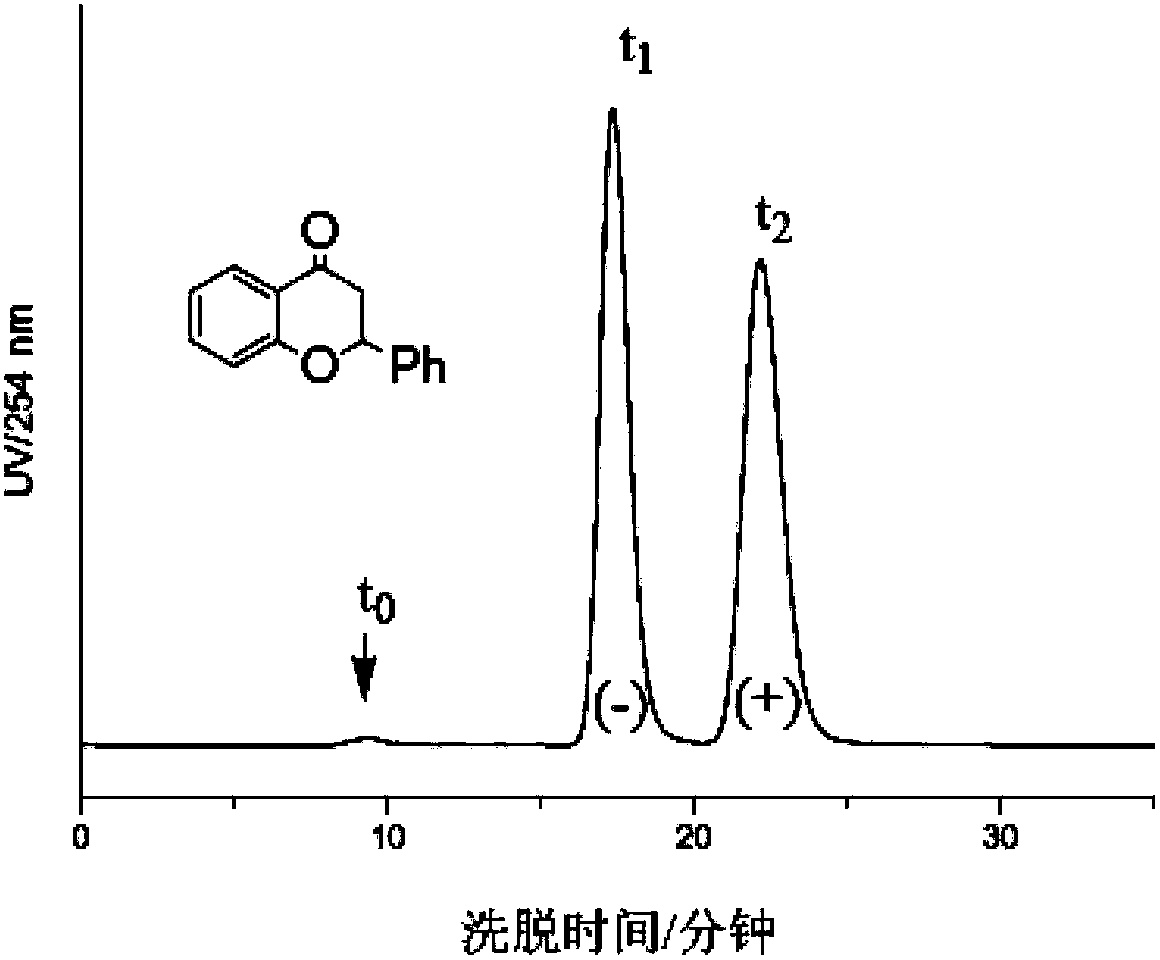
The invention provides a novel chitosan carbanilate-carbamido derivative synthetic method, the method employs chitosan and phenyl isocyanate with different groups to react, and then the hydroxy and amino on chitosan can be completely conversed to the chitosan carbanilate-carbamido derivative of carbamate and carbamido. According to the invention, a coating process is employed to prepare the derivative to a chiral stationary phase, and high performance liquid chromatography is used for resolution of various enantiomers, and the chiral stationary phase has high chiral identification capability.
Owner:DAICEL CHEM IND LTD
Process for the preparation of phenylcarbamates
InactiveUS7385076B2Easy to handleEasy to storeCarbamic acid derivatives preparationOrganic compound preparationHydrogenPhenylcarbamates
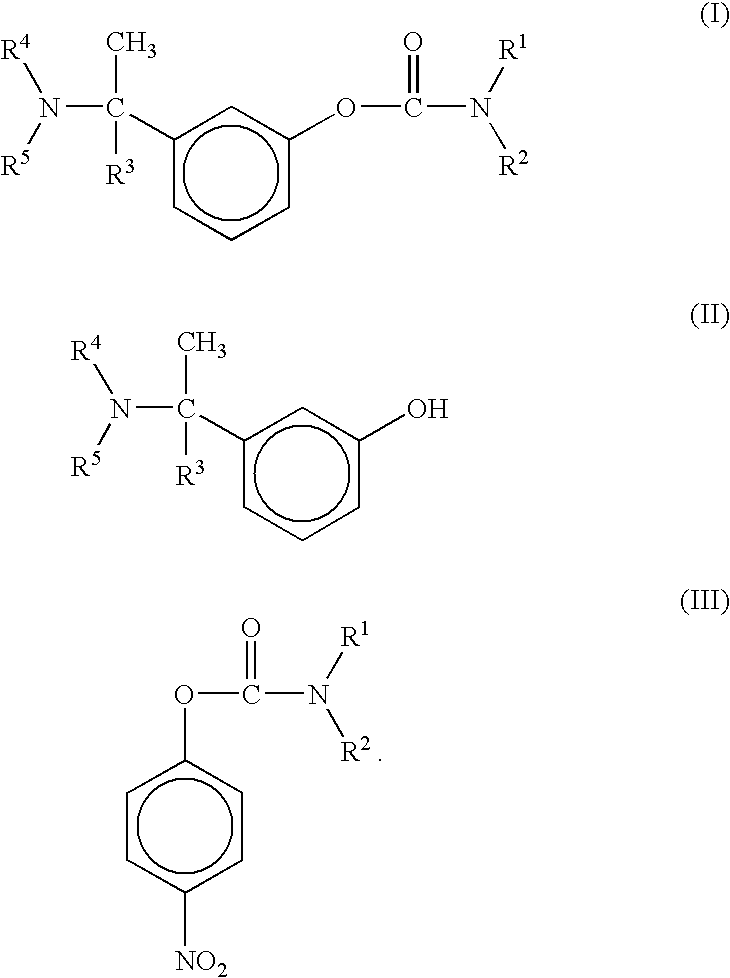
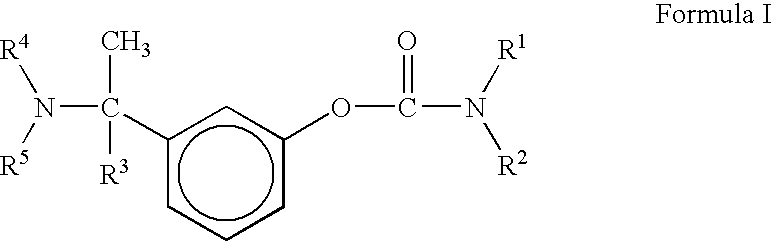
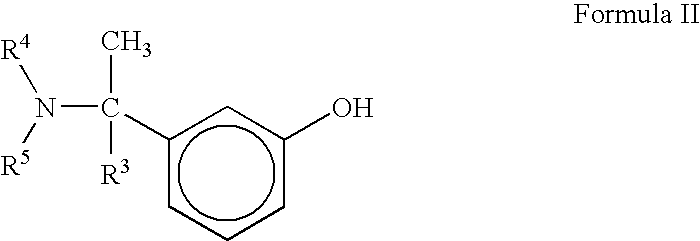
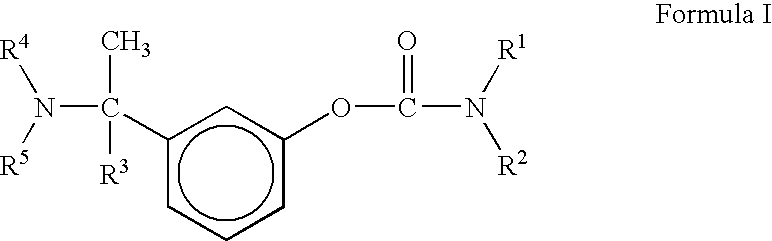
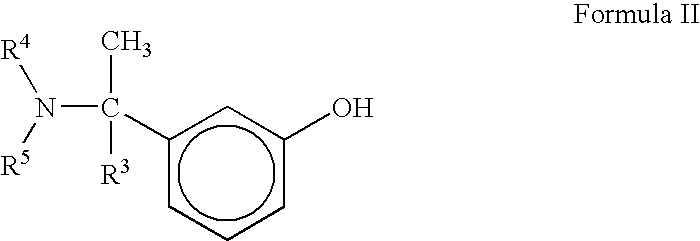
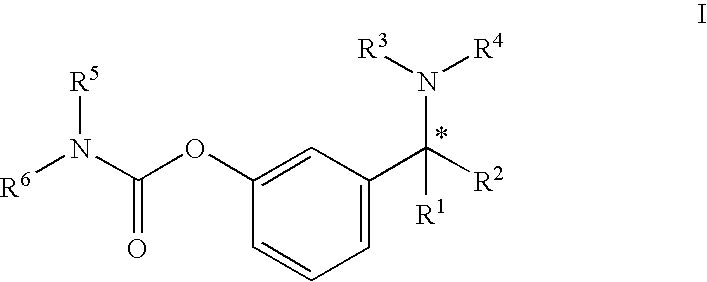
A process for the preparation of compound of formula (I); wherein R1 is hydrogen, linear, branched or cyclic lower alkyl, cyclohexyl, allyl, propargyl or benzyl; R2 is hydrogen, methyl, ethyl or propyl; or R1 and R2 together with the nitogen to which they are attached form a cyclic moiety of three to eight-membered ring, with or without a hetero atom like nitrogen or oxygen; R3 is hydrogen or lower alkyl; R4 and R5 are the same or different and each is a lower alkyl; comprising reacting compound of formula (II); wherein R3, R4 and R5 are as defined above, with compound of formula (III); wherein R1 and R2 are as defined above, in the presence of a base, and further resolving the compound of formula (I) to obtain (S)-isomer of compound of formula (I), substantially free of R-isomer
Owner:SUN PHARMA INDS
Phenylcarbamate compound and muscle relaxant containing the same
A novel phenylcarbamate compound and a pharmaceutical composition containing the same are provided. More specifically, a novel phenylcarbamate compound, a composition for muscle relaxation containing the phenylcarbamate compound as an active ingredient, and a method of muscle relaxation comprising administering a therapeutically effective amount of the phenylcarbamate compound, are provided.
Owner:BIO PHARM SOLUTIONS
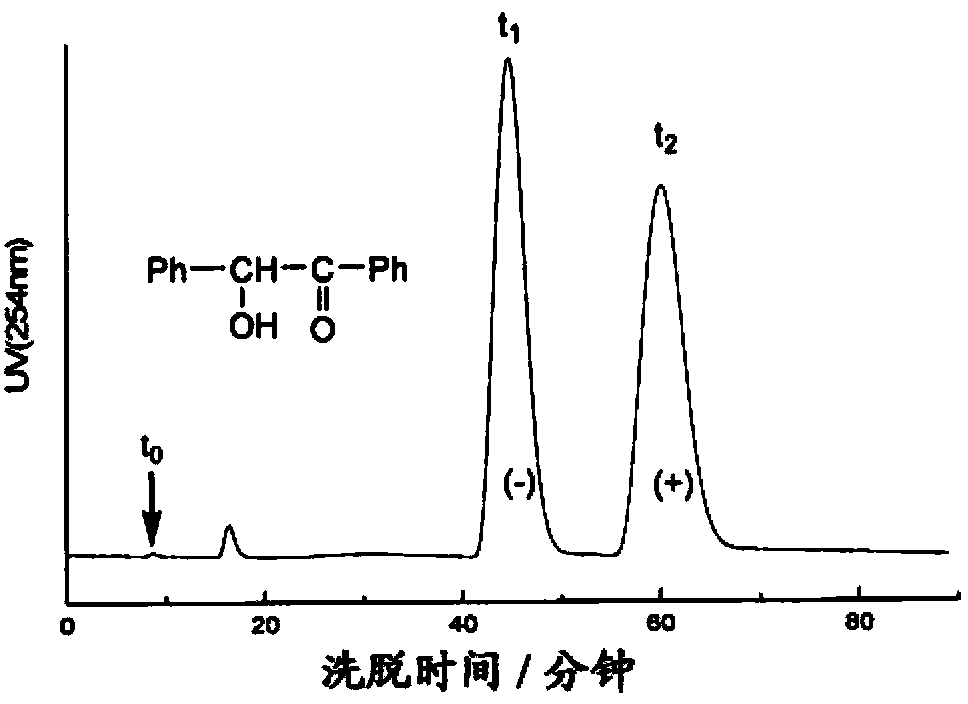
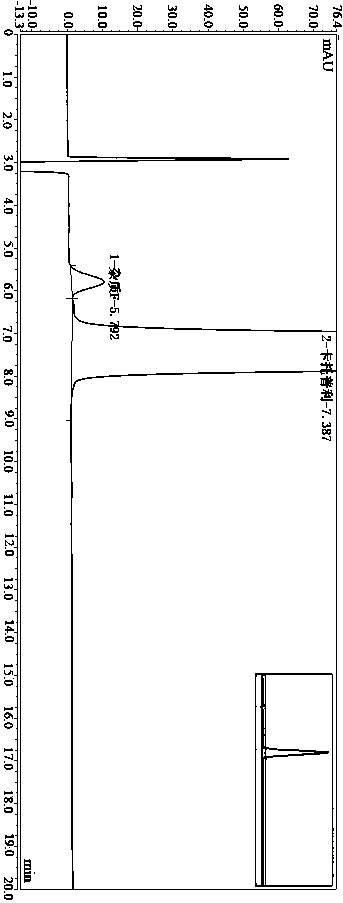
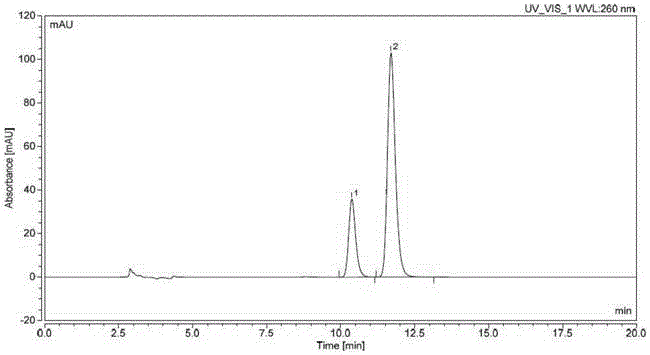
Method for determining impurity F in captopril tablets through high performance liquid chromatography
The invention discloses a method for determining an impurity F in captopril tablets through high performance liquid chromatography and belongs to the technical field of pharmaceutical analysis. Detection is performed under the conditions as follows: an amylase-tris(5-chloro-2-methyl phenyl carbamate) coated chromatographic column is used, normal hexane-absolute ethyl alcohol-trifluoroacetic acid serves as a mobile phase, a volume ratio of the normal hexane to absolute ethyl alcohol to trifluoroacetic acid is 80:20:0.1, a detection wavelength is 215nm, flow velocity is 1ml / min, a column temperature is 35 DEG C and a sample amount is 20[mu]l. A structural formula of the impurity F is as shown in the description. According to the method disclosed by the invention, the content of the impurityF in the captopril tablets can be quantitatively determined, so that the quality of the captopril tablets is effectively controlled. According to the method provided by the invention, the captopril and the impurity F can be proved to be effectively separated in a system suitability solution, and the method has high precision and high separation degree. A signal to noise ratio of a self-contrast solution is more than 10, and if the sample contains the impurity F, the impurity F can be detected.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
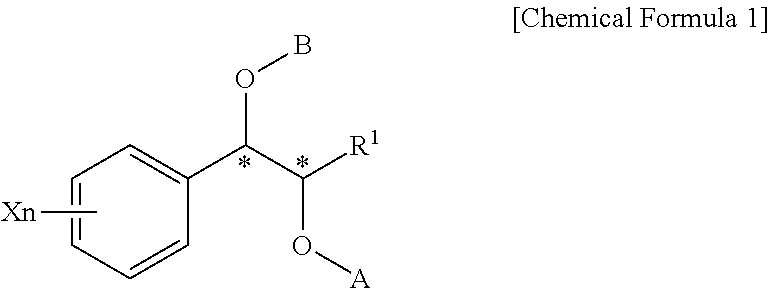
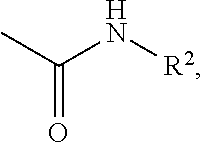
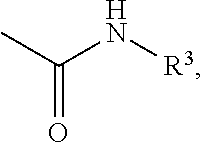
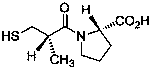
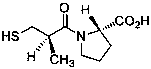
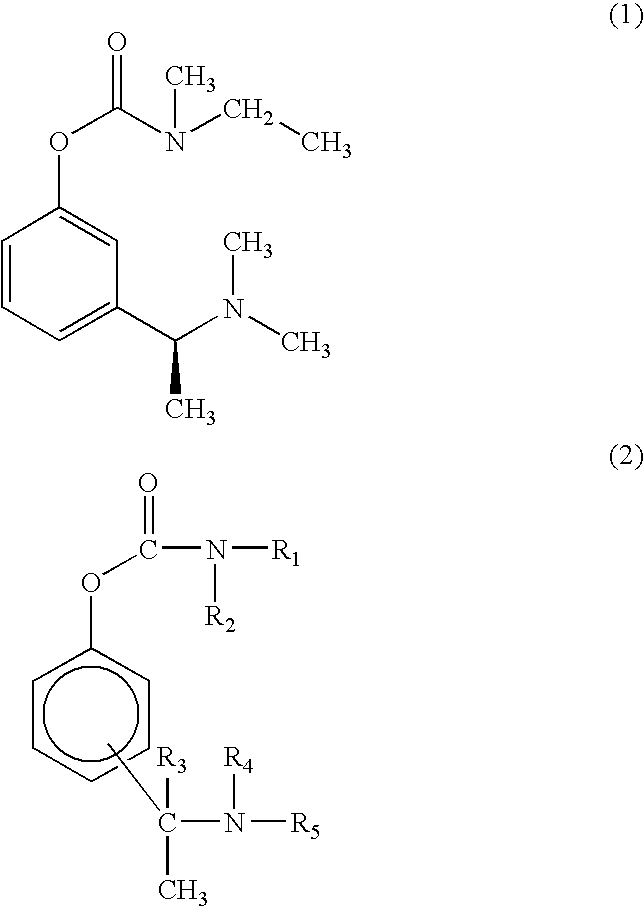
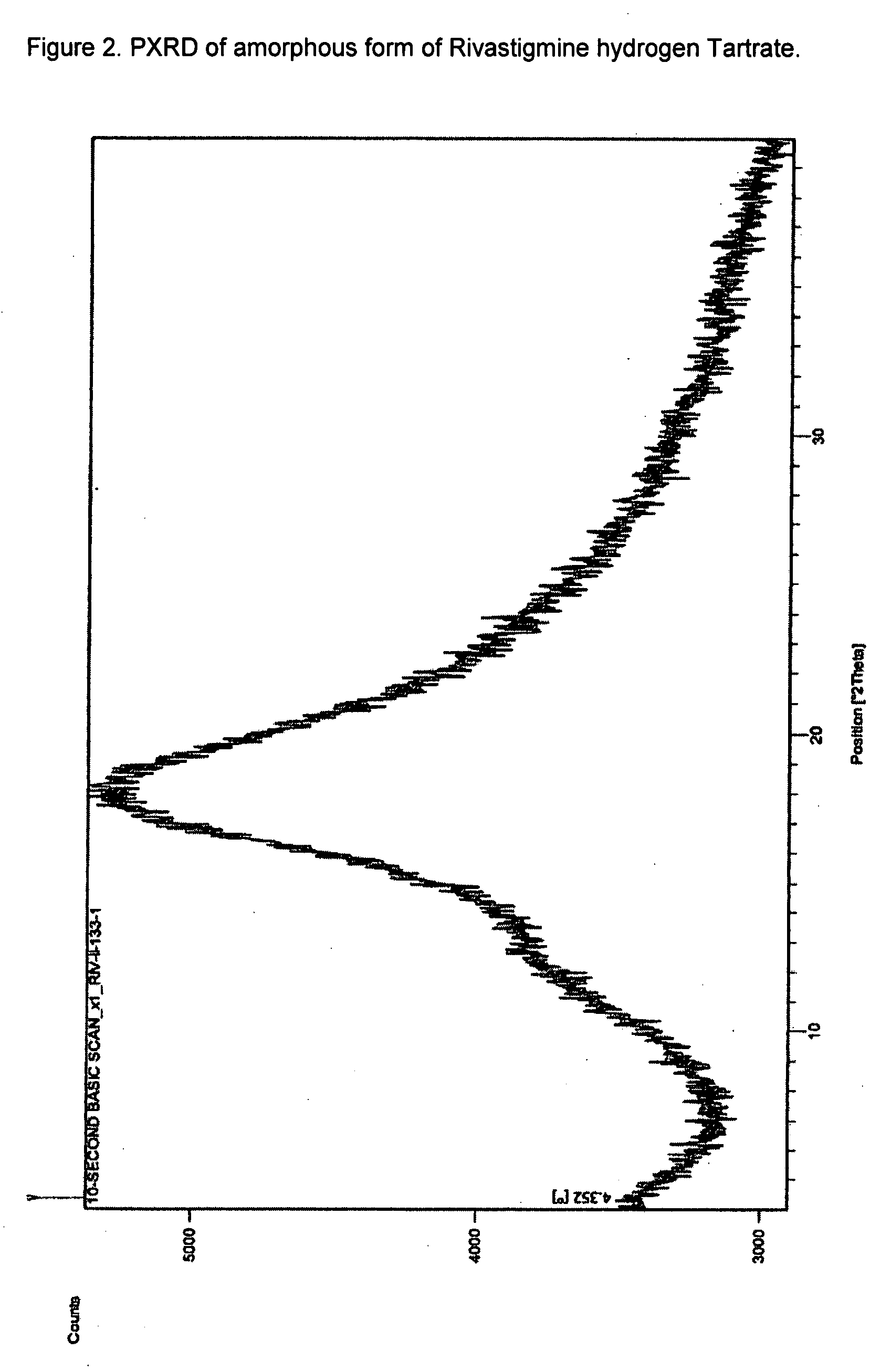
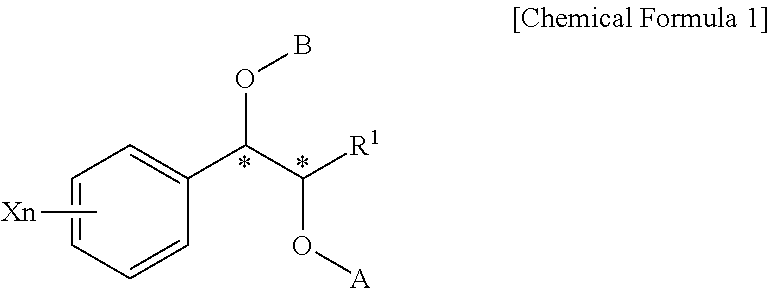
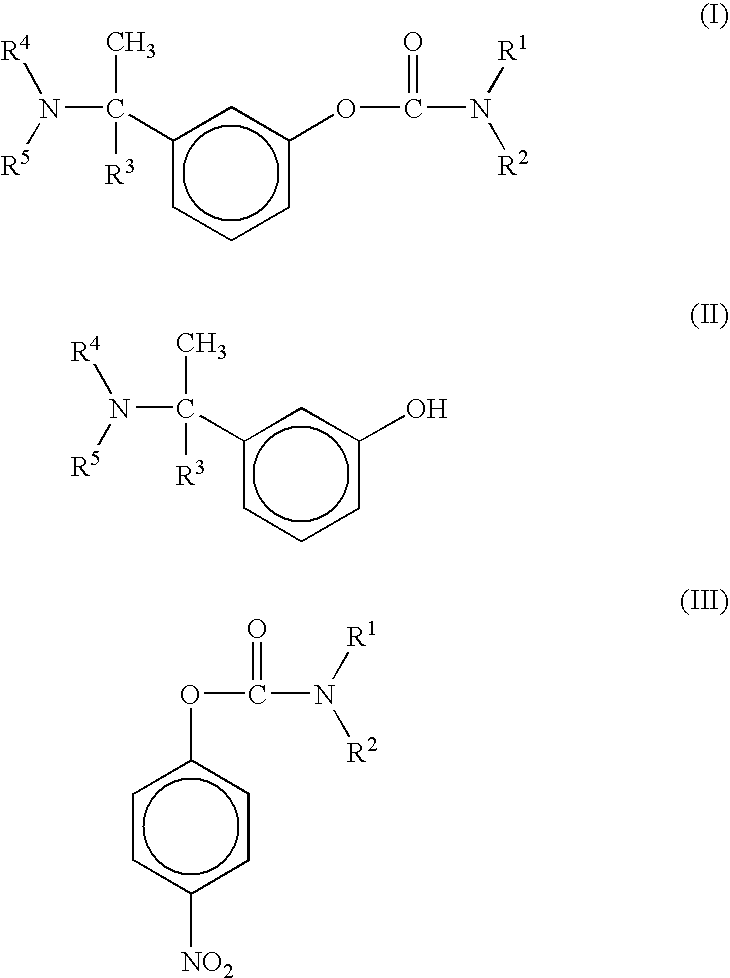
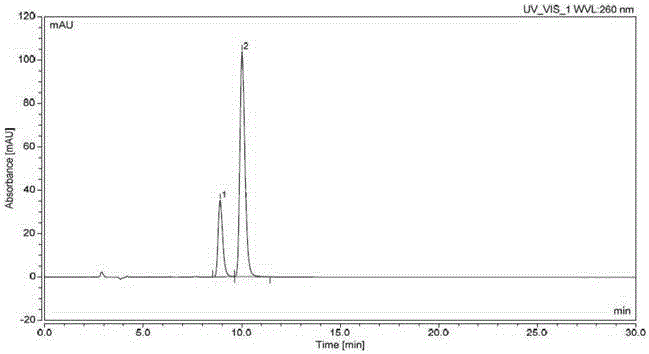
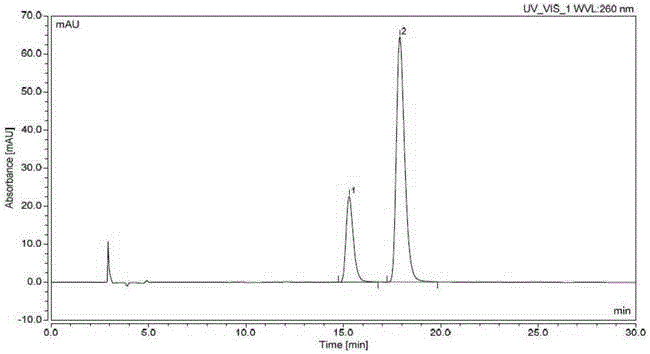
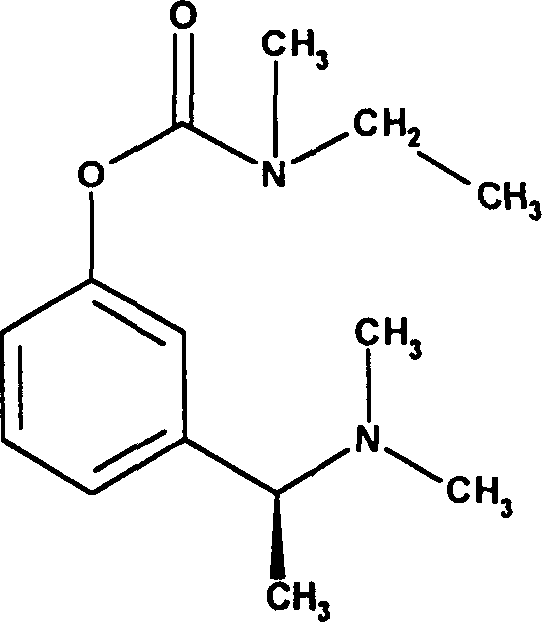
Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration
The invention includes an amount of (3aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl phenylcarbamate for administering to a subject and also a method of preventing or treating neurotoxicity or neurodegenerative processes in a subject in need thereof using the amount thereof.
Owner:QR PHARMA INC
Method for preparing methyl 4-(4'-aminophenylmethylene)phenylaminoformate
InactiveCN101260068AReduce usageAvoid defects that are difficult to remove and affect the performance of downstream productsCarbamic acid derivatives preparationOrganic compound preparationPhenylcarbamatesMethyl carbonate
The invention discloses a preparation method for 4-(4'-aminophenylmethylene) methyl N-phenylcarbamate. Methyl carbonate, diaminodiphenylmet hanes, solvent and catalyst are added into a reactor, the air in the reactor is replaced by aerating nitrogen, the self-pressure lifting is made at the reaction temperature between 150 and 180 DEG C, the reaction is made for 0.5 to 4 hours under the stirring, the mixture obtained after the reaction is filtered, then crystallization solvent is used to make two to three recrystallizations to the filter cake, white crystalloid solids precipitated at 30 DEG C are taken, and the solids are the target product. The method has the advantages of low cost, no pollution and simple preparation.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
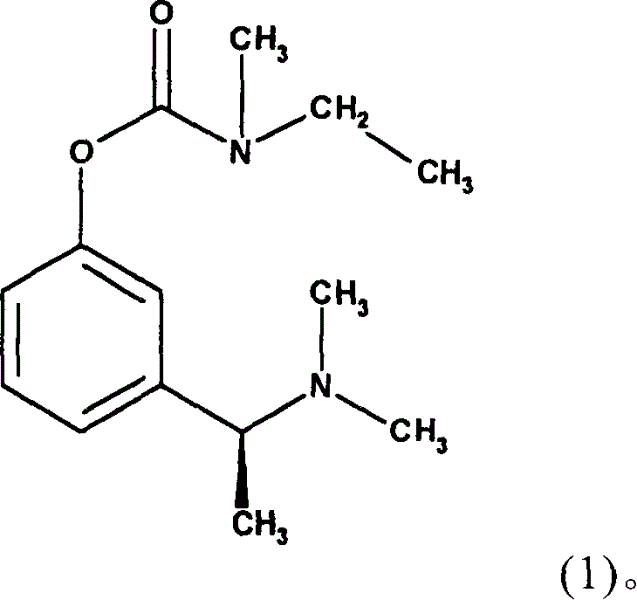
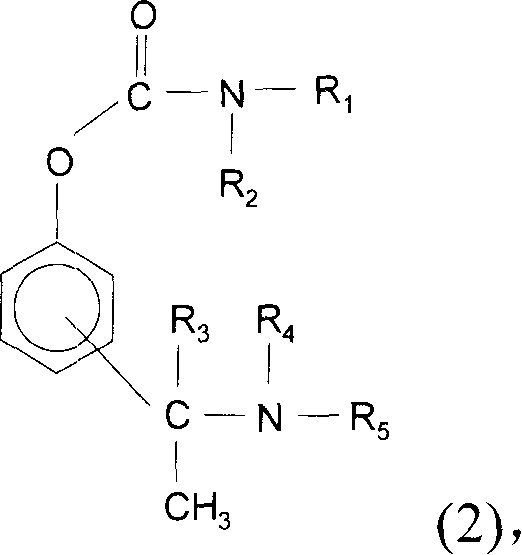
Method for the medicinal prophylaxis of cholinesterase inhibitor intoxication, and active substances and medicaments suitable therefor
InactiveUS20060183796A1Disadvantage is reduced and avoidedAvoid poisoningBiocideAntinoxious agentsCholinesteraseCholinesterase inhibition
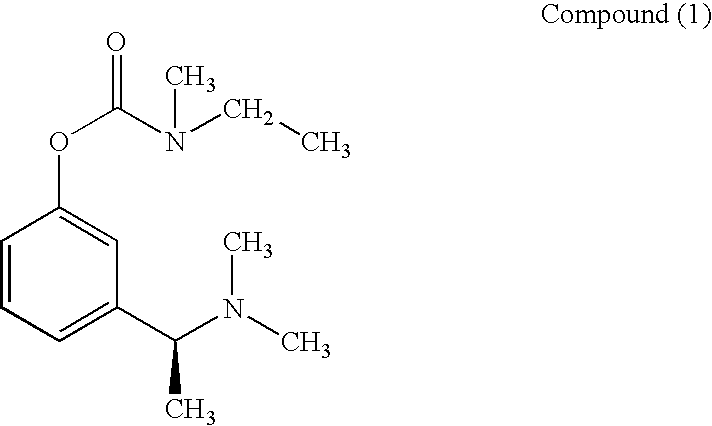
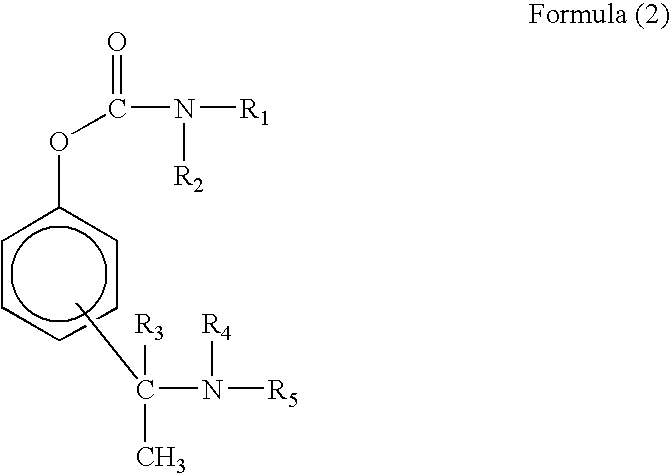
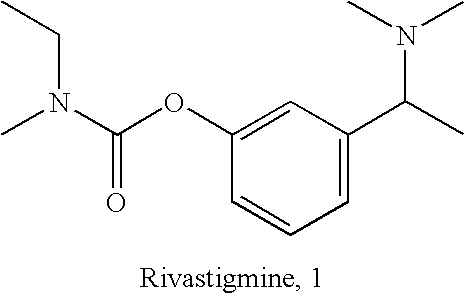
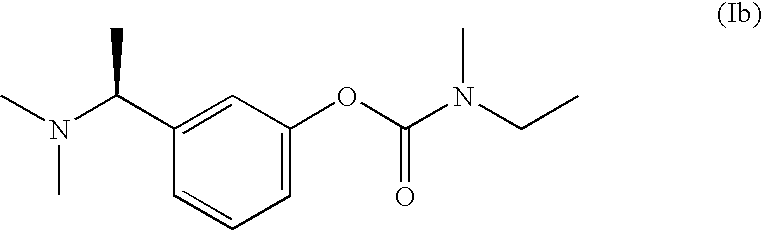
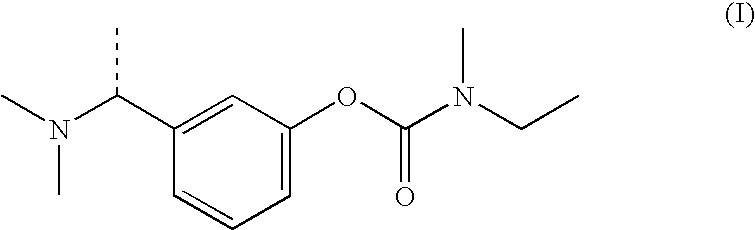
(S)-N-ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate (formula (1)) or at least one active ingredient according to formula (2) for the preventive protection of people from poisoning caused by cholinesterase inhibitors.
Owner:LTS LOHMANN THERAPIE-SYST AG +1
Novel process for the preparation of phenylcarbamates
InactiveUS20080306280A1Less solventShorten production timeBiocideOrganic active ingredientsCarbamateReaction temperature
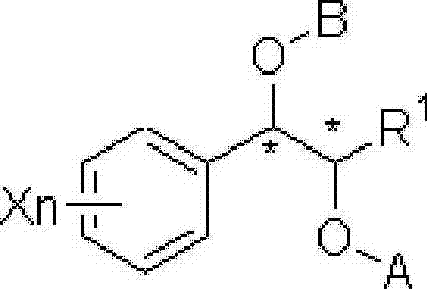
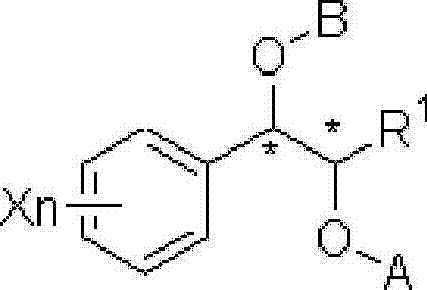
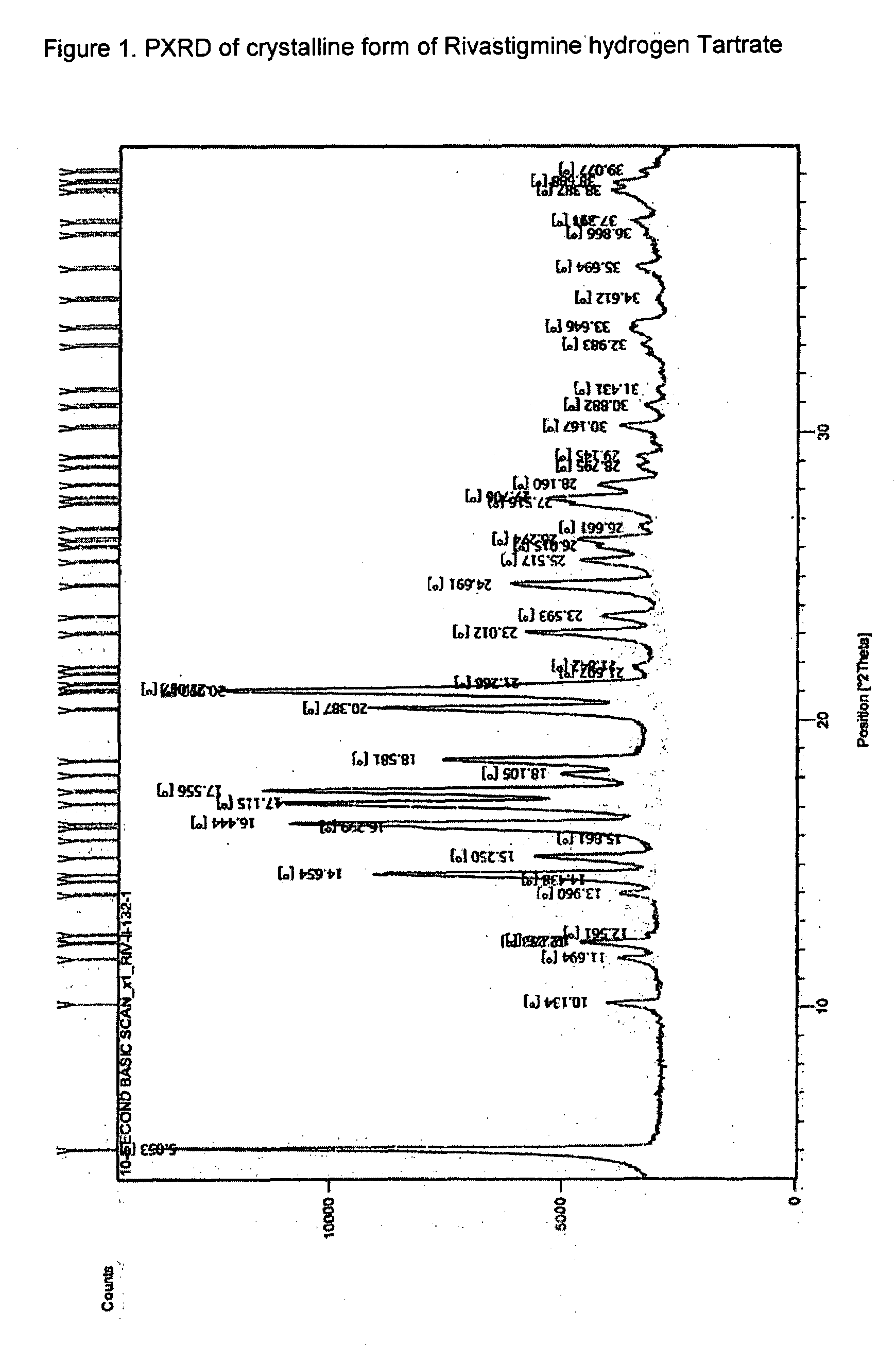
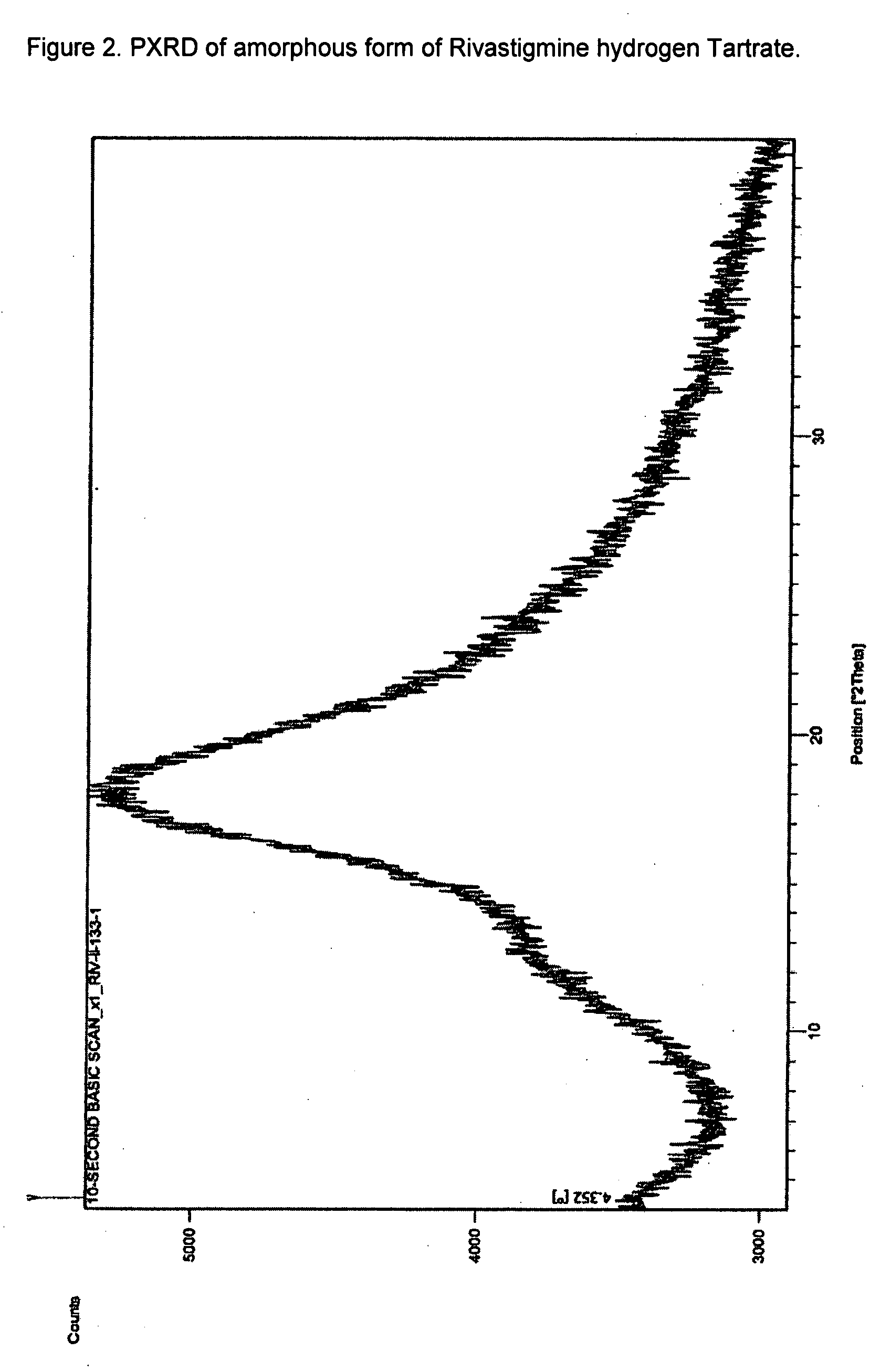
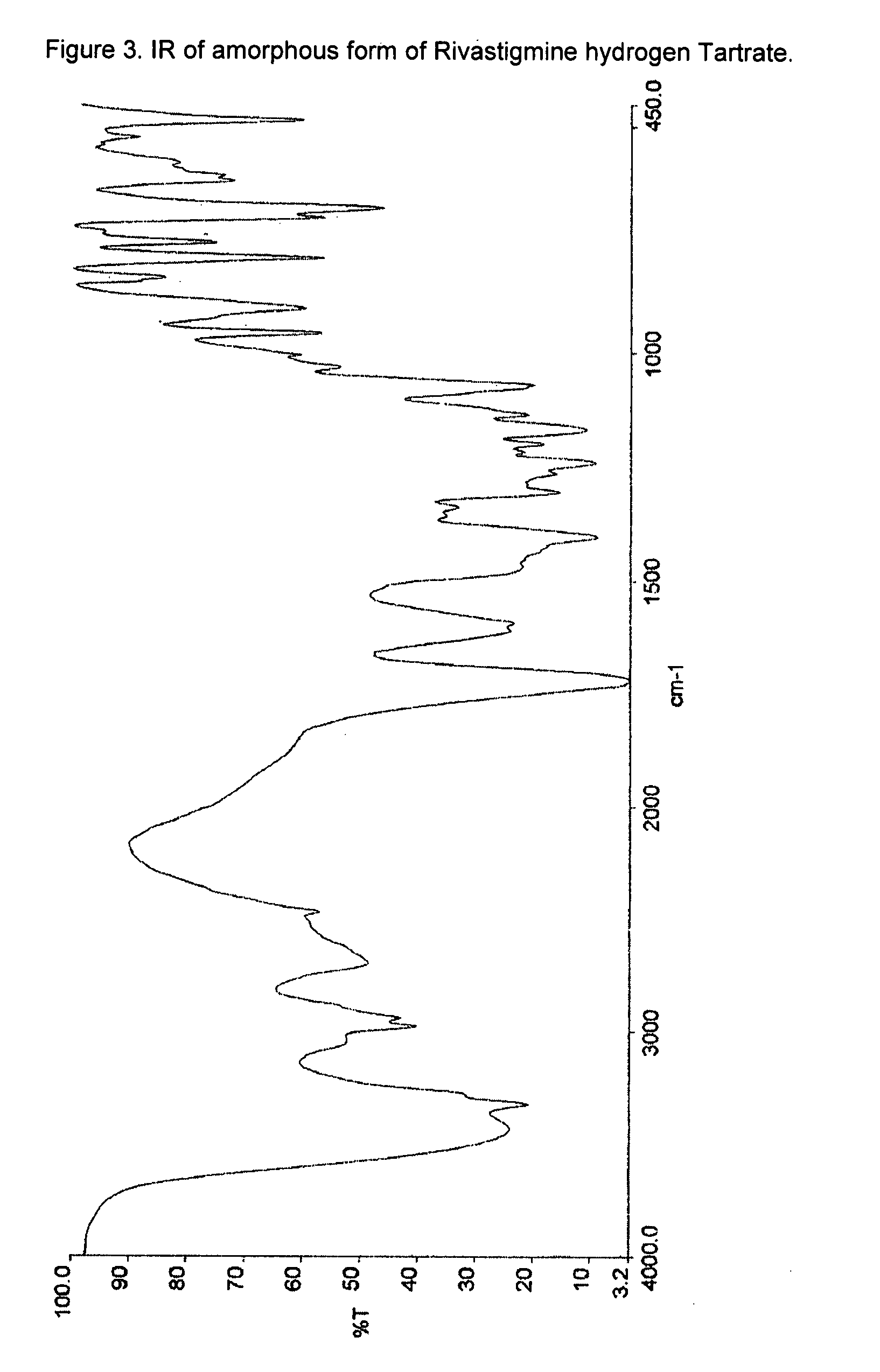
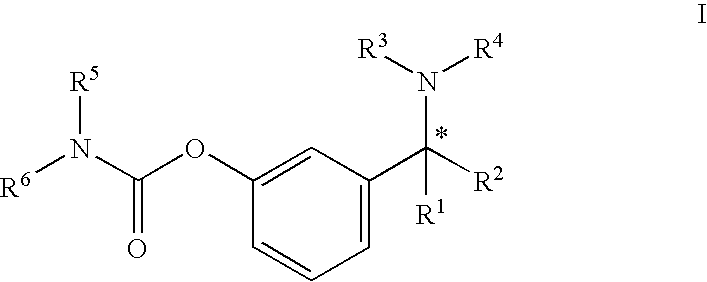
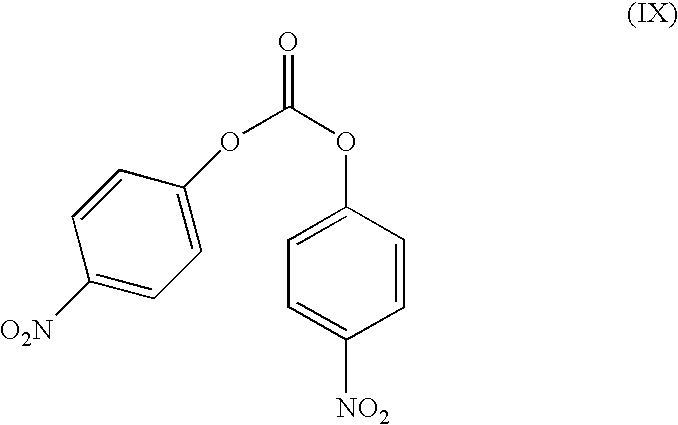
This invention relates to a process for the preparation of an aminoalkyl phenyl carbamate compound of formula 1,wherein R1 and R2 independently are hydrogen or C1-6 alkyl; R3 and R4 are the same or different and each is a lower alkyl; or R3 and R4 together with the nitrogen to which they are attached form a cyclic moiety of a three to eight-member ring, with or without a hetero atom like nitrogen or oxygen; R5 and R6 independently are hydrogen, linear, branched or cyclic C1-6 alkyl, allyl, propargyl or benzyl; or R5 and R6 together with the nitrogen to which they are attached form a cyclic moiety of three to eight member ring, with or without a hetero atom like nitrogen or oxygen; the carbon center marked with “*” is racemic or enantiomerically enriched (R)- or (S)-configuration; and pharmaceutically acceptable addition salts, and crystalline and amorphous forms thereof comprising the steps of:i) converting an amine R5R6NH to a carbamoylimidazolium salt of formula 3wherein R5 and R6 are as defined above; X− is a counterion and R7 is an alkyl or aryl group;ii) reacting in a solvent at a controlled reaction temperature the compound of formula 3 with a compound of formula 4,wherein R1, R2, R3, R4 and “*” are as defined above to give the compound of formula 1; andiii) isolating the compound of formula 1.
Owner:APOTEX PHARMACHEN INC
Method for splitting chlortrimeton enantiomer by using simulated moving bed chromatography
InactiveCN104557682ASimple processContinuous automated productionOrganic chemistryEnantiomerPhenylcarbamates
The invention discloses a method for splitting a chlortrimeton enantiomer by using simulated moving bed chromatography. The method is characterized by comprising the following steps: by adopting a simulated moving bed chromatography system, taking silica gel of amylose-tri(3,5-dimethyl phenylcarbamate) as a filler, and taking a mixed solution of normal hexane and isopropyl alcohol as a mobile phase, splitting the chlortrimeton enantiomer under normal phase conditions, thereby obtaining high-purity R-chlortrimeton and S-chlortrimeton. The simulated moving bed chromatography system has the advantages of continuous production, high degree of automation and high production efficiency.
Owner:JIANGSU HANBON SCI & TECH CO
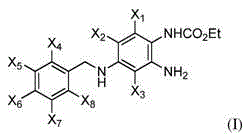
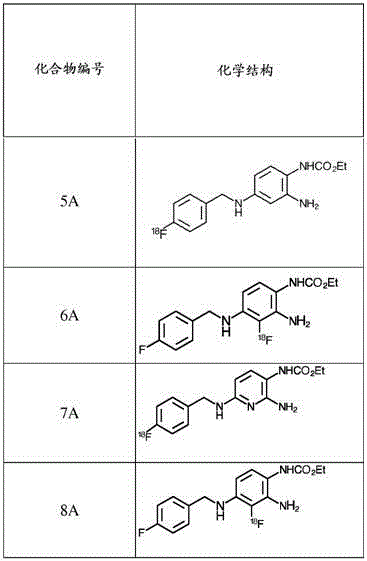
Fluorinated 2-amino-4-(benzylamino)phenylcarbamate derivatives
The invention relates to fluorinated compounds and their use as anti-epileptic, muscle- relaxing, fever-reducing and peripherally analgesically acting medications and as imaging agents. Novel fluorinated 2-amino-4-(benzylamino)phenyl carbamate derivatives of ezogabine and pharmaceutically acceptable salts or solvates thereof and their use are described.
Owner:SCIFLUOR LIFE SCI
Phenylcarbamate compound and muscle relaxant containing the same
A novel phenylcarbamate compound and a pharmaceutical composition containing the same are provided. More specifically, a novel phenylcarbamate compound, a composition for muscle relaxation containing the phenylcarbamate compound as an active ingredient, and a method of muscle relaxation comprising administering a therapeutically effective amount of the phenylcarbamate compound, are provided.
Owner:BIO PHARM SOLUTIONS
Proccess for the preparation of phenylcarbamates
InactiveUS20060293518A1Easy to handleEasy to storeCarbamic acid derivatives preparationOrganic compound preparationHydrogenPhenylcarbamates
A process for the preparation of compound of formula (I); wherein R1 is hydrogen, linear, branched or cyclic lower alkyl, cyclohexyl, allyl, propargyl or benzyl; R2 is hydrogen, methyl, ethyl or propyl; or R1 and R2 together with the nitrogen to which they are attached form a cyclic moiety of three to eight-membered ring, with or without a hetero atom like nitrogen or oxygen; R3 is hydrogen or lower alkyl; R4 and R5 are the same or different and each is a lower alkyl; comprising reacting compound of formula (II); wherein R3, R4 and R5 are as defined above, with compound of formula (III); wherein R1 and R2 are as defined above, in the presence of a base, and further resolving the compound of formula (I) to obtain (S)-isomer of compound of formula (I), substantially free of R-isomer.
Owner:SUN PHARMA INDS
Method for detecting beta-elemene and related substances thereof
ActiveCN112213400AThe detection method is simple and fastGood repeatabilityComponent separationCarbamateBiology
The invention provides a method for detecting beta-elemene and related substances thereof, which comprises the following steps of: performing isocratic elution by taking a polysaccharide derivative ofwhich the surface material is amylose-tris (3-chloro-5-methylphenyl carbamate) as a filler and acetonitrile water as a mobile phase. According to the method, beta-elemene can be effectively separatedfrom adjacent impurities and all the impurities, the reproducibility is good, beta-elemene and related substances thereof can be accurately detected, the problem that beta-elemene and related substances thereof are difficult to separate is effectively solved, and therefore it is guaranteed that the quality of beta elemene is controllable.
Owner:四川弘合生物科技有限公司
O-GlcNAcase-specific inhibitor and substrate engineered by the extension of the N-Acetyl moiety
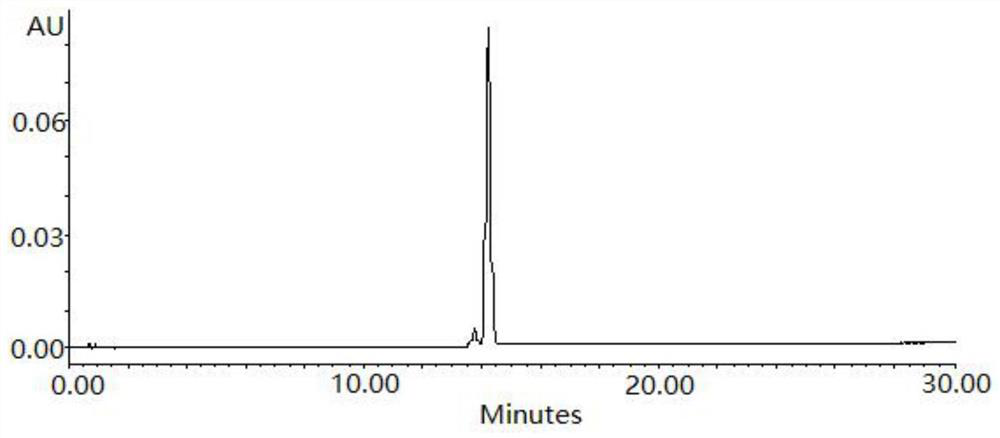
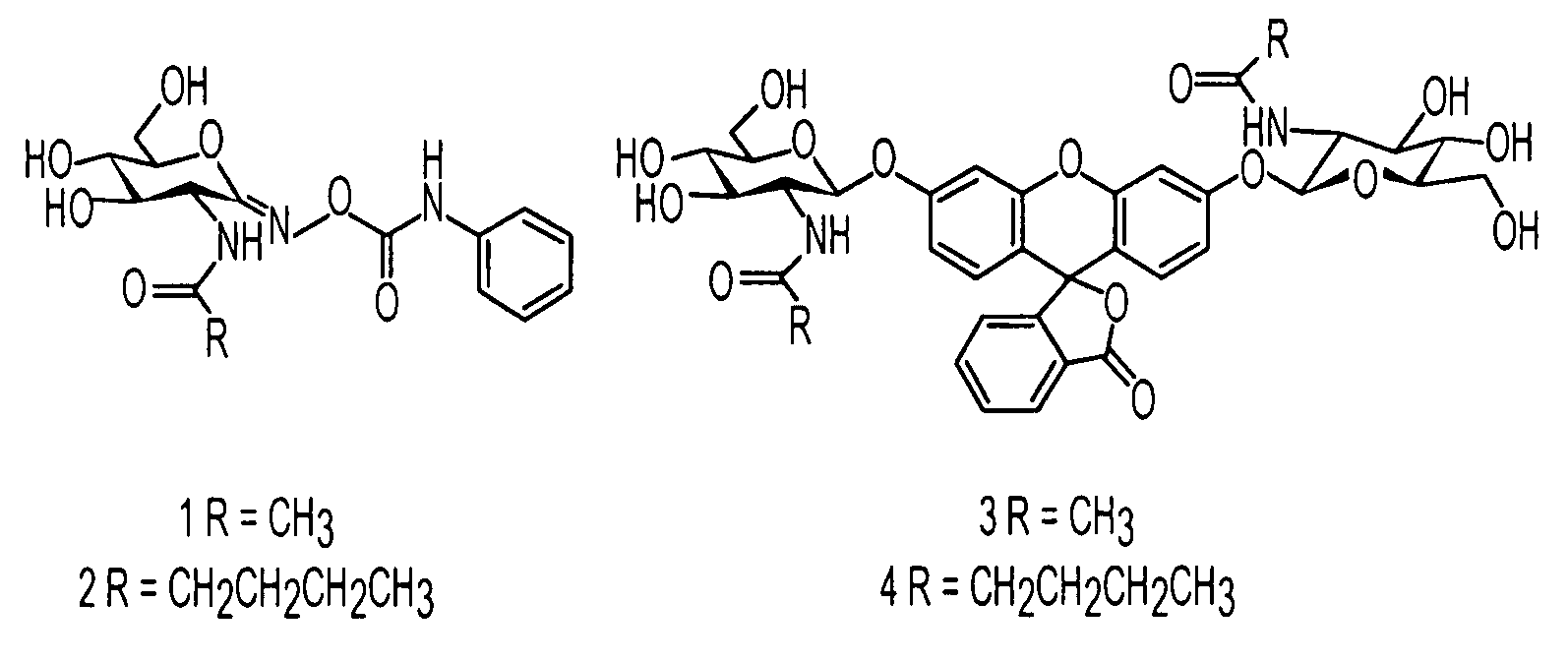
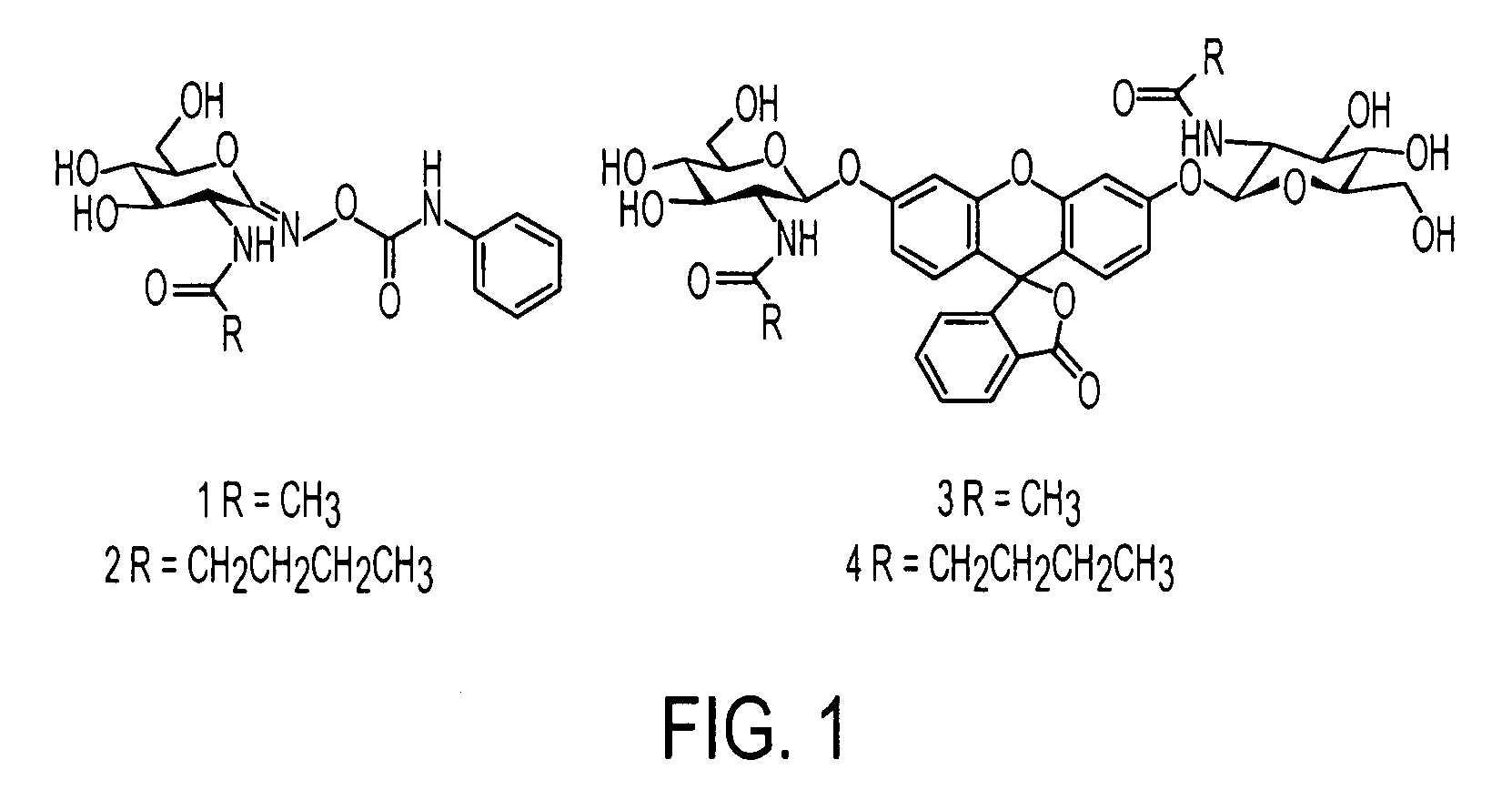
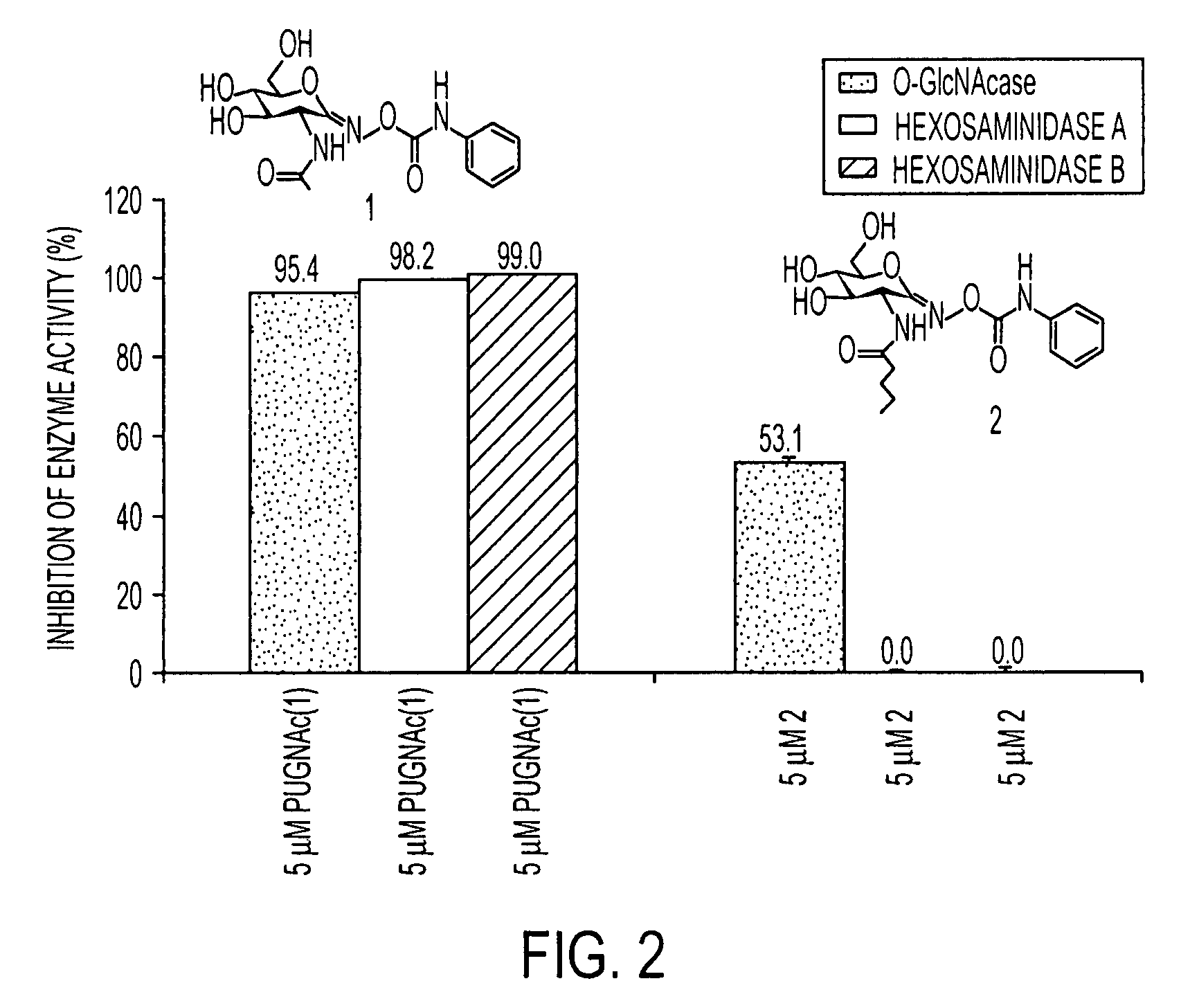
An O-GlcNAcase-specific inhibitor and substrate are engineered by the extension of the N-Acetyl Moiety of O-(2-acet-amido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc). The reagent substrate includes a fluorophor and the inhibitor. This reagent substrate is for high-throughput analysis of O-GlcNAcase within cellular assays and imaging agent for the in vivo analysis of O-GlcNAcase.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA BY THE SEC DEPT OF HEALTH & HUMAN SERVICES THE
Process for the preparation of phenylcarbamates
InactiveUS7884121B2Less solventShorten production timeOrganic active ingredientsBiocideCarbamateAcyl group
This invention relates to a process for the preparation of an aminoalkyl phenyl carbamate compound of formula 1,wherein R1 and R2 independently are hydrogen or C1-6 alkyl; R3 and R4 are the same or different and each is a lower alkyl; or R3 and R4 together with the nitrogen to which they are attached form a cyclic moiety of a three to eight-member ring, with or without a hetero atom like nitrogen or oxygen; R5 and R6 independently are hydrogen, linear, branched or cyclic C1-6 alkyl, allyl, propargyl or benzyl; or R5 and R6 together with the nitrogen to which they are attached form a cyclic moiety of three to eight member ring, with or without a hetero atom like nitrogen or oxygen; the carbon center marked with “*” is racemic or enantiomerically enriched (R)- or (S)-configuration; and pharmaceutically acceptable addition salts, and crystalline and amorphous forms thereof comprising the steps of:i) converting an amine R5R6NH to a carbamoylimidazolium salt of formula 3wherein R5 and R6 are as defined above; X− is a counterion and R7 is an alkyl or aryl group;ii) reacting in a solvent at a controlled reaction temperature the compound of formula 3 with a compound of formula 4,wherein R1, R2, R3, R4 and “*” are as defined above to give the compound of formula 1; andiii) isolating the compound of formula 1.
Owner:APOTEX PHARMACHEN INC
Process for the preparation of phenylcarbamates
A process for the preparation of aminoalkyl phenyl carbamate compounds of Formula I,wherein R1 and R2 independently are hydrogen or a C1-6 alkyl; R3 and R4 are the same or different and each is a C1-6 alkyl; or R3 and R4 together with the nitrogen to which they are attached form a cyclic three to eight membered ring, with or without a heteroatom like nitrogen or oxygen; R5 and R6 independently are hydrogen, linear, branched or cyclic C1-6 alkyl; or R5 and R6 together with the nitrogen to which they are attached form a cyclic three to eight membered ring, with or without a heteroatom like nitrogen or oxygen; the carbon centre designated “*” can be racemic or enantiomerically enriched in the (R)- or (S)- configuration; and pharmaceutically acceptable acid addition salts thereof.
Owner:APOTEX PHARMACHEN INC
Process for making aminoalkylphenyl carbamates and intermediates therefor
InactiveUS7531684B2Carbamic acid derivatives preparationOrganic compound preparationCarbamatePhenylcarbamates
Owner:SYNTHON BV
Diclazuril enantiomer chiral chromatographic separation analysis method
ActiveCN109001326AChiral Chromatographic Separation RealizationEasy to separateComponent separationCelluloseChromatographic separation
The invention discloses a diclazuril enantiomer chiral chromatographic separation analysis method. The separation analysis method comprises the following steps of carrying out pretreatment on a chiralstationary phase, taking normal hexane, trifluoroacetic acid and lower alcohol as a mobile phase, and carrying out liquid phase chromatography separation analysis, wherein the pretreatment refers towashing of the chiral stationary phase by using the mobile phase, the washing time is not less than 2 hours, and the washing flow rate is not greater than 0.5mL.min-1; the chiral stationary phase is bonding type to parachloro-phenylcarbamoyl beta-cyclodextrin, coating type cellulose tri(3, 5-dimethyl phenylcarbamate) or coating type straight-chain starch tri(3, 5-dimethyl phenylcarbamate); the lower alcohol is ethanol, n-propyl alcohol, isopropanol or n-butyl alcohol; and the volume ratio of normal hexane to the lower alcohol is 50-80: 50-20. According to the chiral chromatographic separationanalysis method provided by the invention, the chiral stationary phase is pretreated, and the normal hexane, the trifluoroacetic acid and the lower alcohol are used as the mobile phase, so that the chiral chromatographic separation of the diclazuril enantiomer is realized.
Owner:广州研创生物技术发展有限公司
3-cyanopyridine production method
The present invention discloses a 3-cyanopyridine production method, which belongs to the technical field of fine chemical production, raw material molar ratio is as follows: 3-methylpyridine:ammonia:oxygen:homemade catalyst is 1: 4-14: 12-40: 1.5-2.5; and key points of the production method are as follows: 1, selection of the raw material ratio; 2, selection of appropriate temperature, to be more specific, the temperature is as low as possible under possible conditions;3, selection of reaction time; and 4, selection of a catalyst, to be more specific, the catalyst is selected for more full reaction to obtain a highest yield. The 3-cyanopyridine production method has the advantages of simple production process, low production cost and no pollution, the product yield can reach 70%, the content can reach 90%; the main use of the product is as follows: 1. 3-cyanopyridine is used as a dye intermediate; 2. the 3-cyanopyridine is used as a pesticide pymetrozine intermediate, rodenticide pyridyl phenylcarbamates and mieshujing intermediate; 3. and the 3-cyanopyridine is used as a pharmaceutical intermediate, pigment intermediate, resin intermediate, and the like, and also used as an important chemical intermediate.
Owner:JIANGSU WEUNITE FINE CHEM CO LTD
Method for preparing and detecting intermediate and corresponding isomer of afatinib
ActiveCN106442793AGuaranteed accuracyGuaranteed optical purityComponent separationCelluloseIsocratic elution
The invention discloses a method for simultaneously detecting a key intermediate (formula 2) and a corresponding isomer (formula 3) of afatinib. According to the method, isocratic elution is performed on a high performance liquid chromatograph; a chromatographic column which takes cellulose-tris(3,5-dimethyl phenylcarbamate) as a filling agent is adopted; a flow phase is a mixed solution of n-hexane, isopropyl alcohol and acetonitrile. Through the method, the key intermediate (formula 2) and the corresponding isomer (formula 3) of the afatinib can be effectively separated and detected, and the separation degree can reach 2.0 or higher.
Owner:SHINEWAY PHARMA GRP LTD
Method for the medicinal prophylaxis of cholinesterase inhibitor intoxication, and active substances and medicaments suitable therefor
The present invention discloses (S)-N-ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenylcarbamate (1) or one or Various active substances are used for the prophylactic protection of individuals against intoxication by cholinesterase inhibitors.
Owner:LTS LOHMANN THERAPIE-SYST AG +1
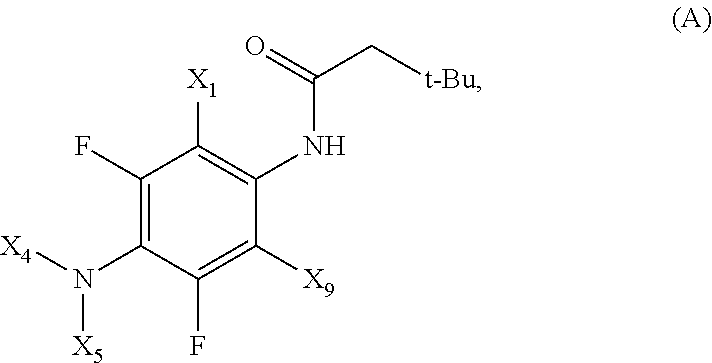
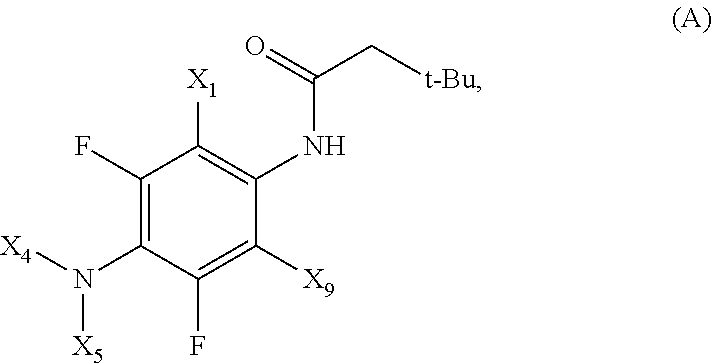
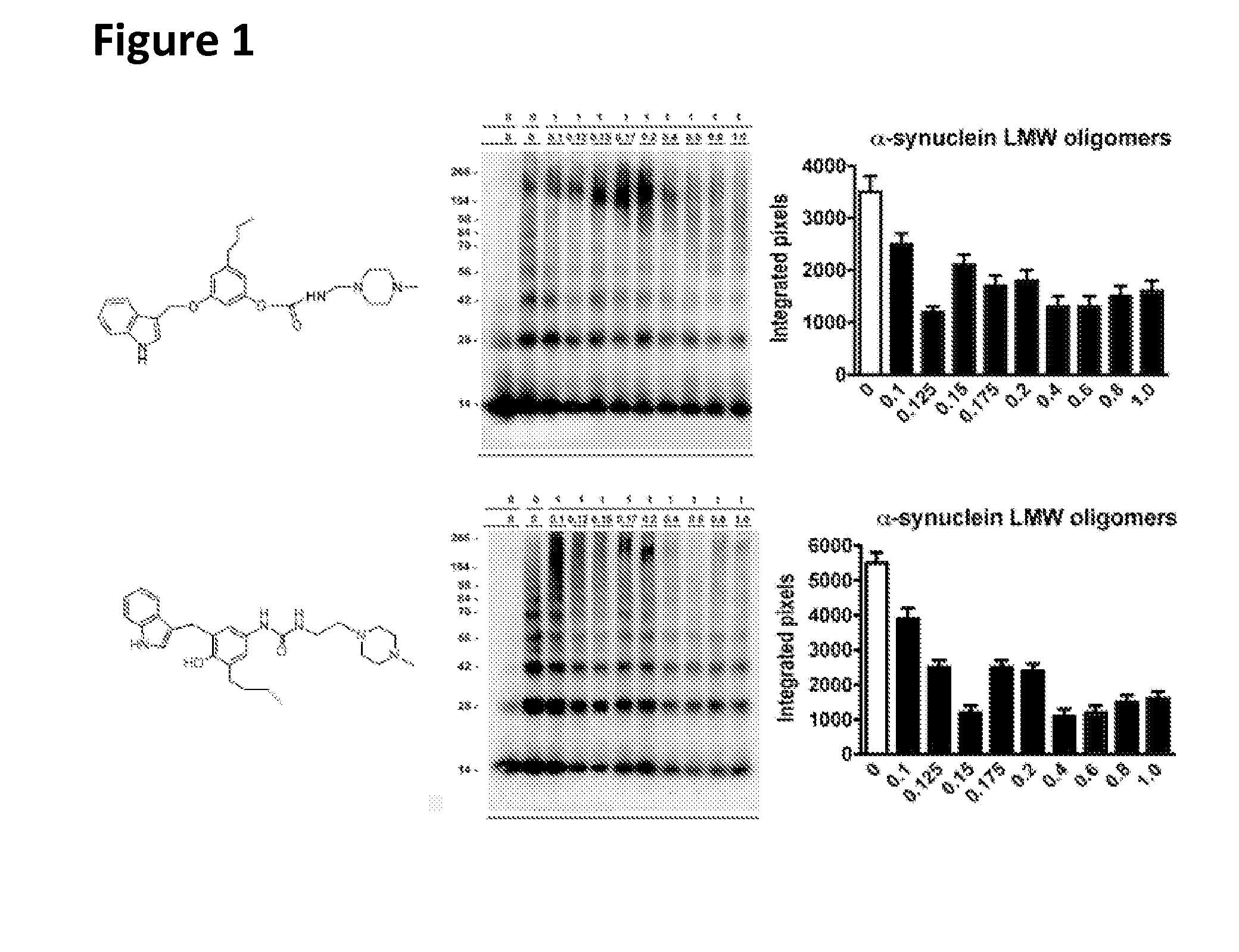
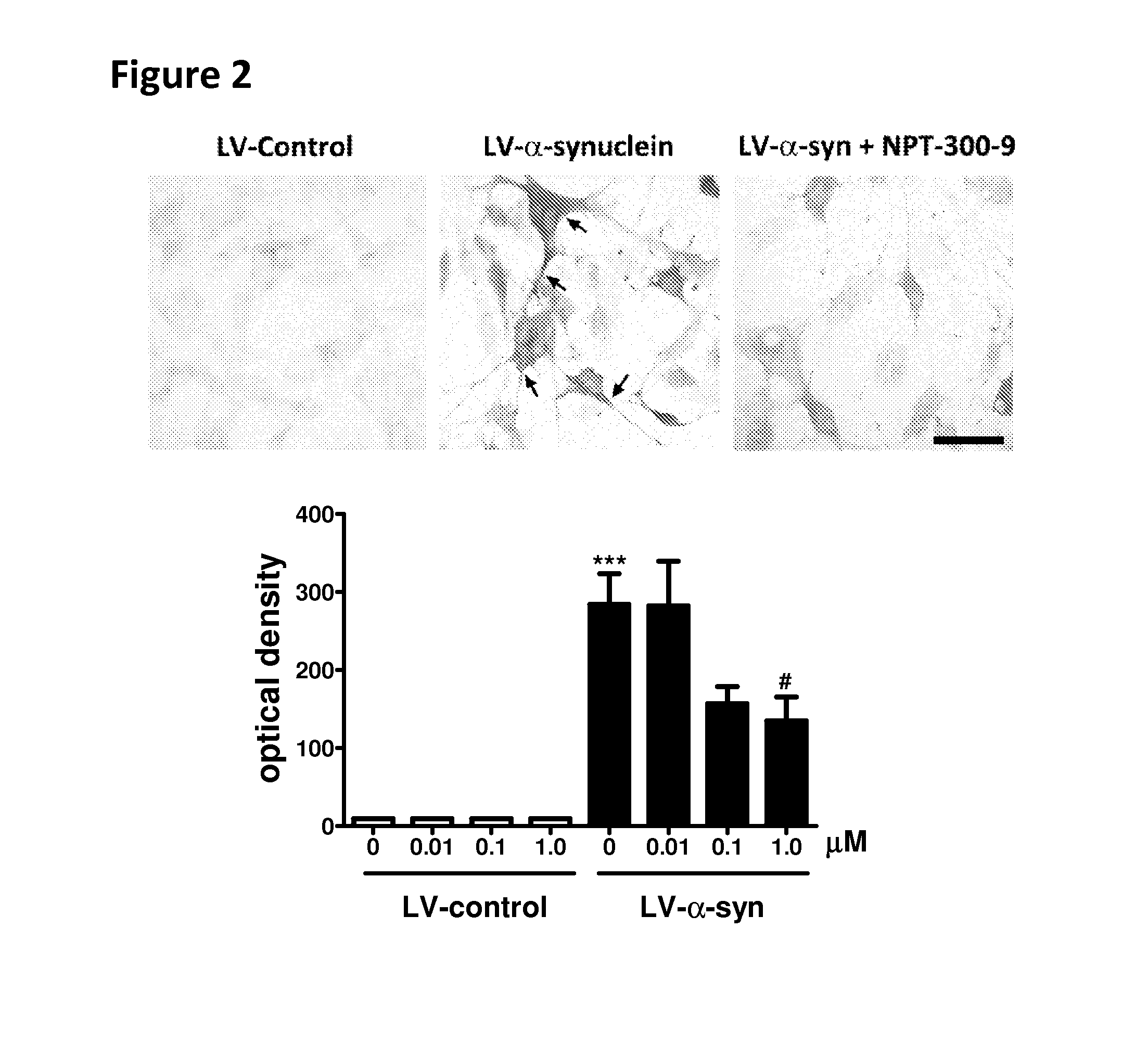
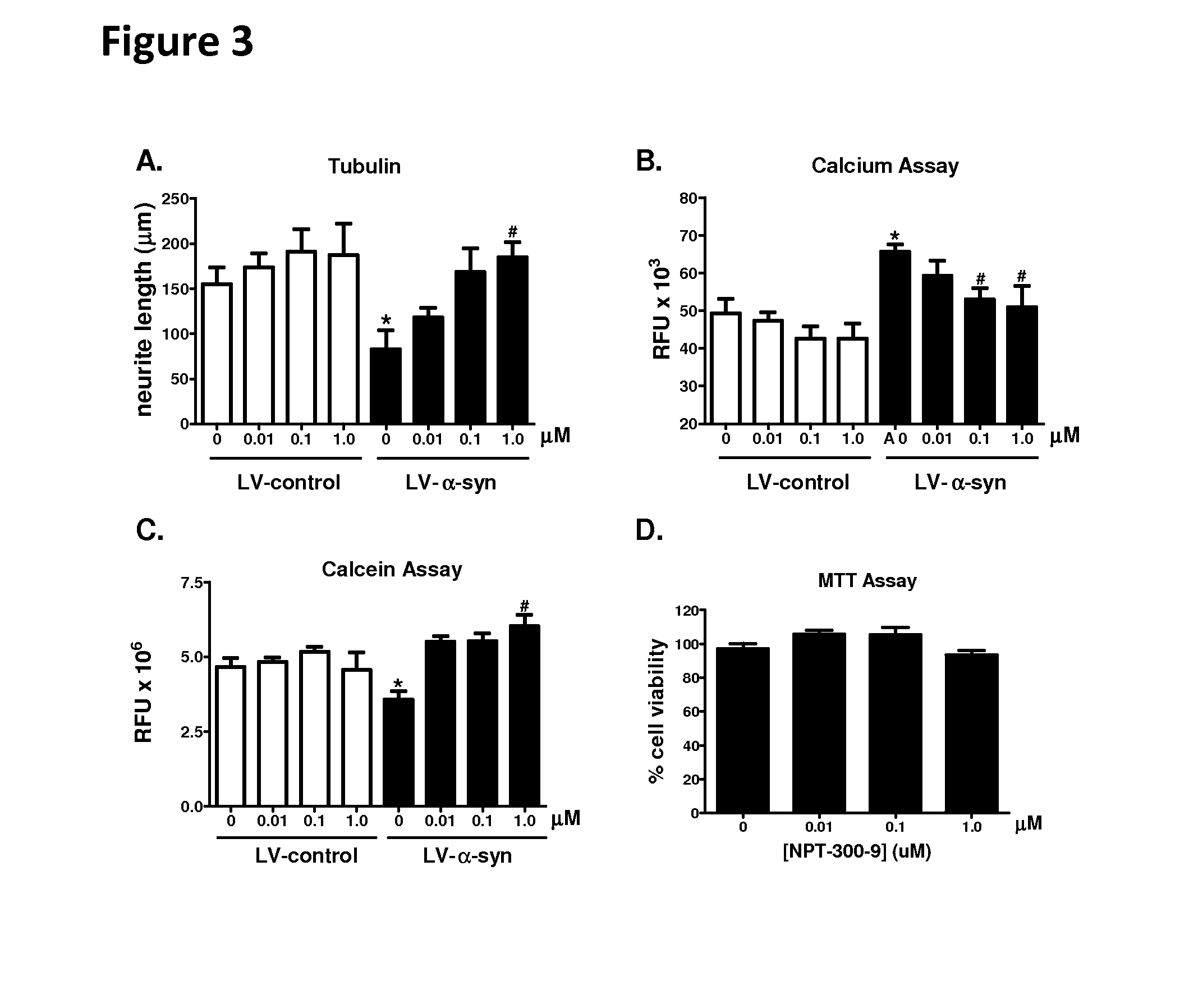
Phenyl-urea and phenyl-carbamate derivatives as inhibitors of protein aggregation
The present invention relates to certain phenyl-urea and phenyl-carbamate derivatives, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, and multiple system atrophy.
Owner:UCB PHARMA SRL
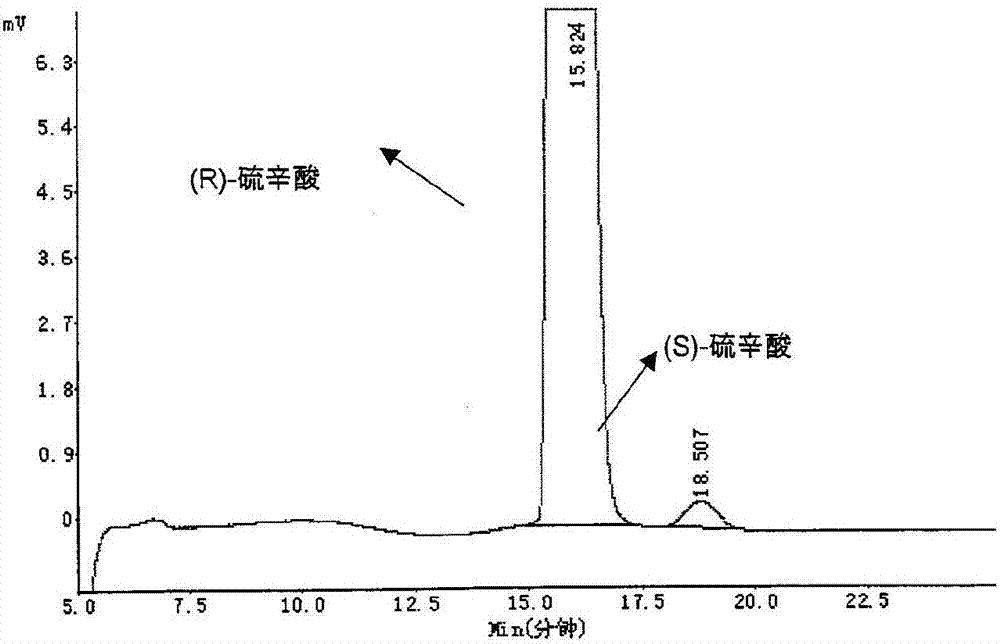
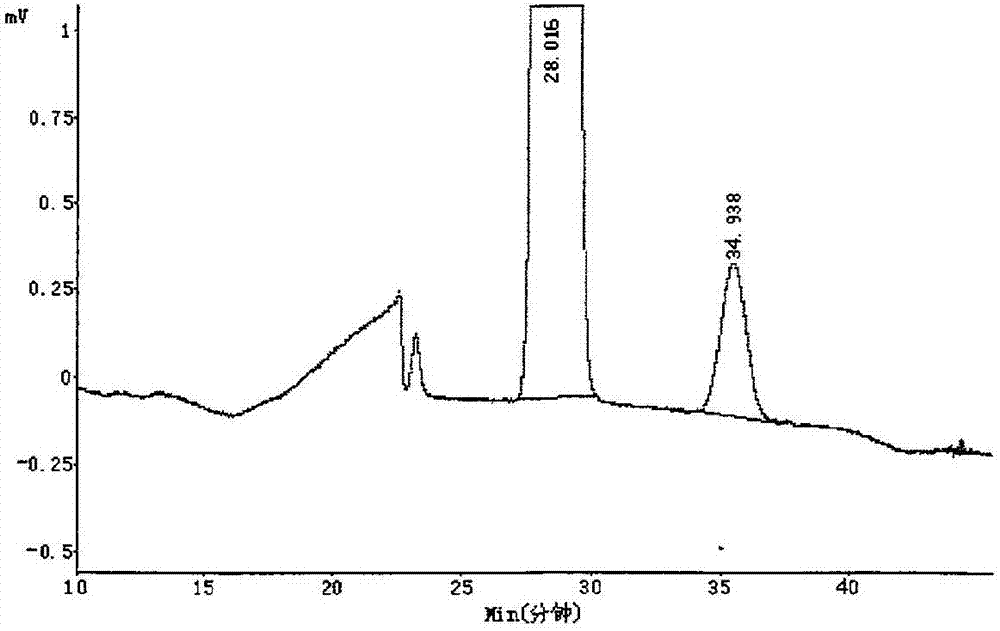
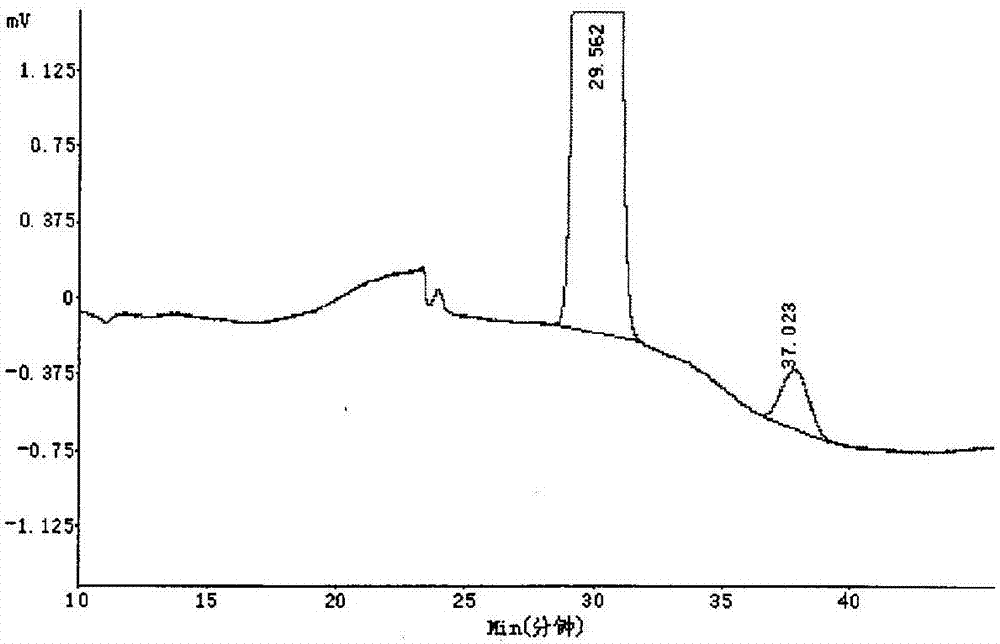
Method of measuring content of optical isomers in (R)-lipoic acid
InactiveCN106959348AEfficient separationAccurately measure contentComponent separationAlkaneColumn temperature
The invention relates to a method of measuring content of optical isomers in (R)-lipoic acid. In the method, a silica gel chiral column coated by amylose-tri-((S)-alpha-methyl-phenylcarbamate) is used, a mixed solvent of an alkane solvent, an alcohol solvent and an organic acid modifier is used as a mobile phase, optical isomers are separated under control of the flow rate and the column temperature of the silica gel chiral column and are detected through an ultraviolet detector, wherein the wavelength of the ultraviolet detector is controlled, so as to obtain the content of the optical isomers of R-lipoic acid. Through adoption of the method, two optical isomers of (R)-lipoic acid can be separated, and the content of each optical isomer of (R)-lipoic acid can be measured accurately, so that the content of optical isomers in R-(+)-lipoic acid tromethamine salt crude drug and preparation can be measured, a foundation is laid for quality analysis and quality control of the R-(+)-lipoic acid tromethamine salt crude drug and the preparation thereof, and the medicine security and effectiveness are ensured.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration
The invention includes an amount of (3aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl phenylcarbamate for administering to a subject and also a method of preventing or treating neurotoxicity or neurodegenerative processes in a subject in need thereof using the amount thereof.
Owner:QR PHARMA INC
Detection method of chiral isomers in carfilzomib
The invention relates to a normal-phase high-performance liquid chromatography detection method for detecting three chiral isomers (enantiomers, diastereoisomers F and G) in carfilzomib. Liquid chromatography adopts a chromatographic column with cellulose-tris(4-chloro-3-methylphenylcarbamate) bonded silica gel as filler; mobile phase is n-hexane-isopropanol-ethanol, n-hexane The volume ratio of ‑isopropanol‑ethanol is (87~91):(6~4):(7~5). This detection method has high sensitivity, good specificity, high accuracy and good durability, and can effectively control card Fezomi quality.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Method for detecting isomer in briviact injection by using high performance liquid chromatography
The invention belongs to the technical field of medicine quality determination, and particularly relates to a method for detecting an isomer in a briviact injection by using high performance liquid chromatography. In order to solve the problem that the method for accurately detecting the isomer in the briviact injection is lacked in the prior art, the technical scheme of the invention is as follows: (1) taking the briviact injection, and diluting the briviact injection to serve as a test solution; (2) detecting the test solution by adopting a high performance liquid chromatography, and judging whether isomer impurities exist or not according to impurity peaks in a map; the chromatographic conditions of the high performance liquid chromatography are as follows: a mobile phase adopts phosphate buffer solution-acetonitrile, and a chromatographic column adopts amylose-tri (3-chloro-5-methyl phenyl carbamate) bonded silica gel as a filling agent. By adopting the chromatographic conditions, the accurate detection of the isomer in the briviact injection can be realized.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
A kind of hplc detection method of pitavastatin isopropyl tert-butyl ester diastereomer
The invention relates to the technical field of drug detection, and specifically discloses an HPLC detection method for diastereomers of pitavastatin isopropyl tert-butyl ester. The chromatographic conditions of the HPLC detection method of the diastereomer of pitavastatin isopropyl tert-butyl ester are: chromatographic column: amylose-tris(3-chloro-5-methylphenylcarbamate) is a filler; mobile phase: water and acetonitrile with a volume ratio of 35:60‑70; column temperature: 28‑32℃; detection wavelength: 243‑247nm; flow rate: 0.2‑1mL / min; elution method: isocratic wash take off. The HPLC detection method for diastereomer of pitavastatin isopropyl tert-butyl ester provided by the invention can realize the simultaneous detection of two diastereomers in pitavastatin isopropyl tert-butyl ester, and the precise High accuracy, high reproducibility and good reproducibility provide a reliable guarantee for improving and controlling the quality of pitavastatin isopropyl tert-butyl ester and the final product pitavastatin calcium.
Owner:SHIJIAZHUANG NO 4 PHARMA
Method for measuring DD-captopril and LD-captopril in captopril bulk drug by using HPLC method
The invention discloses a method for measuring DD-captopril and LD-captopril in a captopril bulk drug by using an HPLC method. A normal phase liquid chromatography is adopted, a chromatographic columnadopts an amylose-tri(5-chlorine-2-methylphenyl carbamate) coating filler, a mobile phase is n-hexane-isopropanol-ethanol- trifluoroacetic acid, the volume ratio of which is 85: 10: 5: 0.1, the detection wavelength is 215 nm, the flow rate is 0.95 to 1.05 mL / min, the column temperature is 28 to 32 DEG C, and the injection volume is 10 muL. A system applicability test is performed according to themethod provided by the invention, the captopril, DD-captopril and LD-captopril can be effectively separated, the method is high in specificity, good in resolution and high in precision, the signal-to-noise ratio of an own contrast solution is greater than 10, and the contents of the DD-captopril and LD-captopril in the captopril bulk drug can be quantitatively measured, thereby effectively controlling the quality of captopril raw materials.
Owner:SHANDONG XINHUA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00000.png)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00001.png)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo [2,3-b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/cd33b0b4-60f7-4f68-8bb9-142763977a45/US20120225922A1-20120906-D00002.png)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/0c714c65-27e1-4378-a2f7-5f87ba92baa1/US20150374664A1-20151231-C00001.PNG)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/0c714c65-27e1-4378-a2f7-5f87ba92baa1/US20150374664A1-20151231-C00002.PNG)
![Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration Effective Amounts of (3aR)-1,3a,8-Trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3 b]indol-5-yl Phenylcarbamate and Methods of Treating or Preventing Neurodegeneration](https://images-eureka.patsnap.com/patent_img/0c714c65-27e1-4378-a2f7-5f87ba92baa1/US20150374664A1-20151231-D00001.PNG)