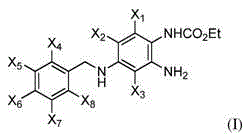
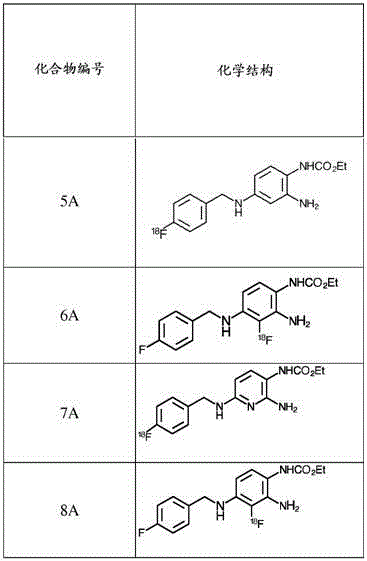
Fluorinated 2-amino-4-(benzylamino)phenylcarbamate derivatives
A technology of compounds and compositions, applied in the direction of palladium organic compounds, platinum group organic compounds, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] Begin with Compound A or E in the preparation outlined in Schemes 1A and 2A. Both are commercially available from chemical suppliers.
[0115] In Step 1 of Scheme 1A, the fluorine atom adjacent to the nitro group of compound A is converted to an amino group to form compound B. For example, compound A can be treated with methanolic ammonia to form compound B. In step 2, the remaining fluorine atom is coupled to the benzylamino compound to form compound C. For example, the fluorine atom of compound B can use Et 3 N, I 2 Coupling with 4-fluorobenzylamine with DMSO forms compound C. In step 3, the nitro group of compound C is reduced and carbamate is formed to provide compounds of formula I. For example, the nitro group of compound C can be reduced using zinc powder and ammonium chloride in methanol. Carbamate formation can be carried out using ethyl chloroformate. In some cases, Compound B is commercially available, in which case the synthetic scheme starts at Step ...
Embodiment 1
[0298] Example 1. Experimental Procedures and Characterization of Compounds
Embodiment 1A
[0299] Example 1A: Synthesis of ethyl (2-amino-3-fluoro-4-((4-fluorobenzyl)amino)phenyl)carbamate (Compound 1A in Table 1)
[0300]
[0301] 2,3-Difluoro-6-nitroaniline (2)
[0302] A solution of 1,2,3-trifluoro-4-nitrobenzene (1) (1.00 g, 5.64 mmol, 1.00 equiv) in methanolic ammonia (1.5 mL) was placed in a microwave vial and heated under microwave to 70°C for 90 minutes. The solvent was evaporated in vacuo to give a crude product which was purified by silica gel column chromatography (EtOAc / Hexane 1:49) to give compound 2 (0.350 g, 35.6%) as a yellow solid. TLC: 10% EtOAc / hexane (R f : 0.10); 1 H NMR (500 MHz, DMSO- d 6 ): δ 7.94-7.91 (m, 1H), 7.51 (s, 2H), 6.75-6.70 (m, 1H); LC-MS: m / z= 173 (M + -1) 3.15 (99.8% purity) at room temperature.
[0303] 2-Fluoro-N1-(4-fluorobenzyl)-4-nitrobenzene-1,3-diamine (3)
[0304] To a stirred suspension of compound 2 (0.100 g, 0.570 mmol, 1.00 equiv) in dry DMSO (4.6 mL) was added 4-fluorobenzylamine (0.210 g, 1.72 mmol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com