Method for measuring DD-captopril and LD-captopril in captopril bulk drug by using HPLC method
A raw material drug, topril technology, applied in the direction of measuring devices, material separation, analysis of materials, etc., can solve the problem of unchecked captopril isomers, DD-captopril and LD-captopril Research and other issues, to achieve the effect of strong specificity, good separation, and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
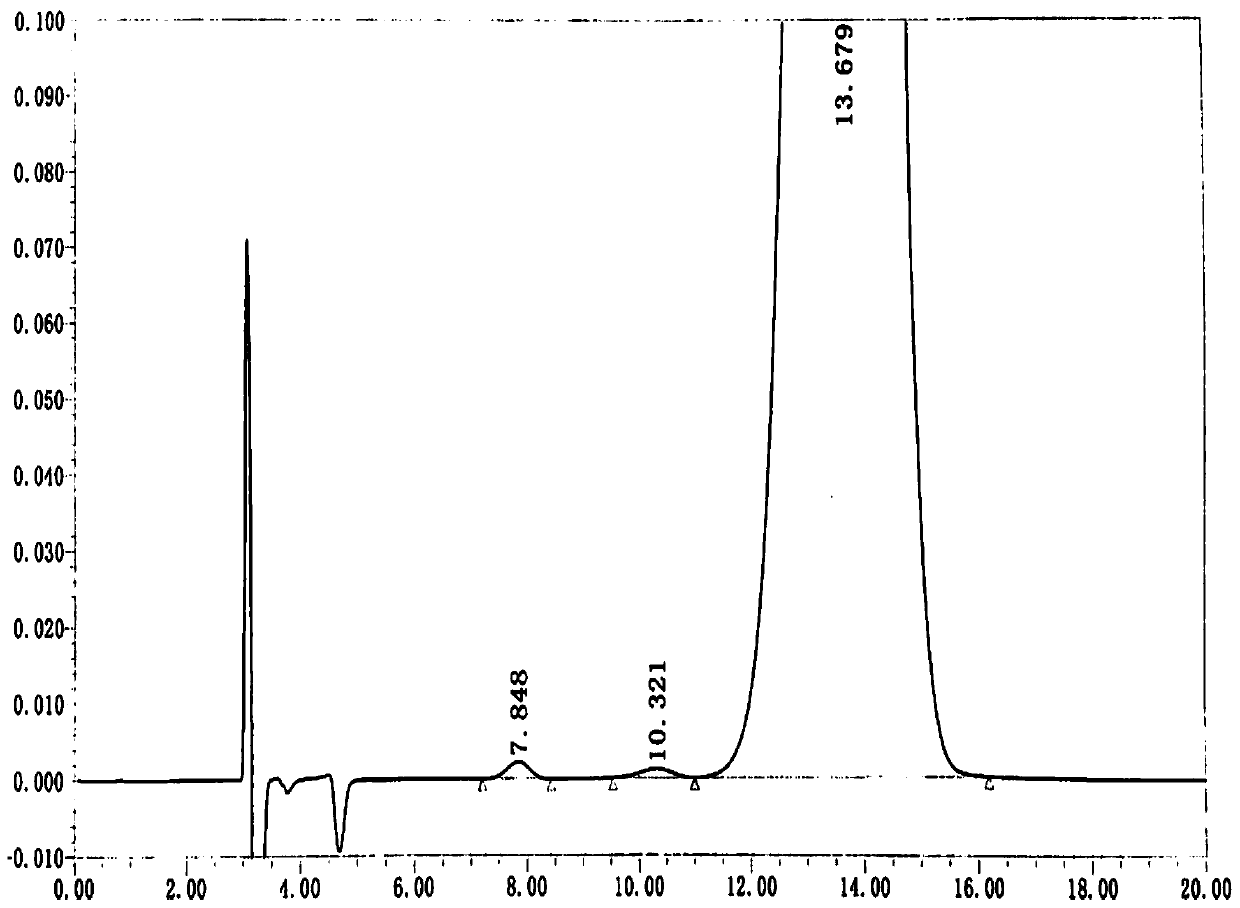
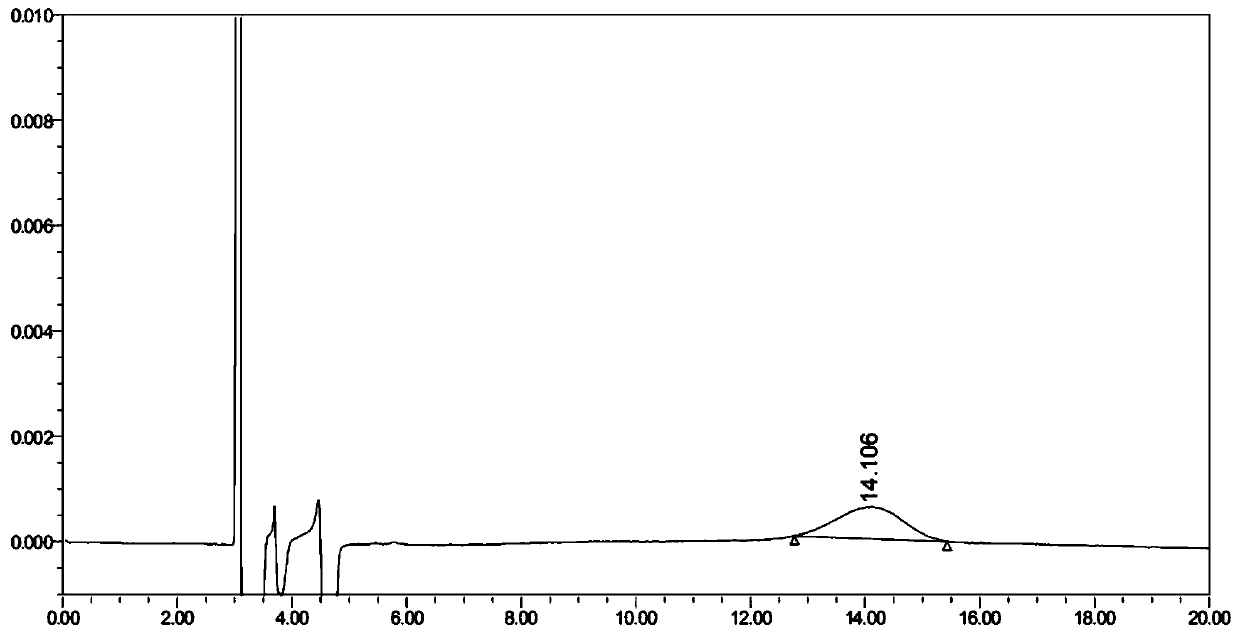
[0045] The content determination of DD-captopril and LD captopril in embodiment 1 captopril crude drug
[0046] (1) Preparation of impurity reference substance stock solution
[0047] Mixed impurity reference substance stock solution: Accurately weigh about 5mg of DD-captopril and about 5mg of LD-captopril into the same 50mL measuring bottle, add absolute ethanol to dissolve and dilute to the mark, shake well, and obtain.
[0048] Single impurity reference substance stock solution: Accurately weigh about 2 mg each of DD-captopril, LD-captopril, impurity F, and captopril reference substance, place them in 20mL measuring bottles, add absolute ethanol to dissolve And dilute to the mark, shake well, that is.
[0049] (2) Preparation of system suitability solution
[0050] Accurately weigh about 50 mg of the captopril reference substance into a 10mL measuring bottle, add precisely pipetted 0.5mL mixed impurity reference substance stock solution, add absolute ethanol and dilute to...
Embodiment 2
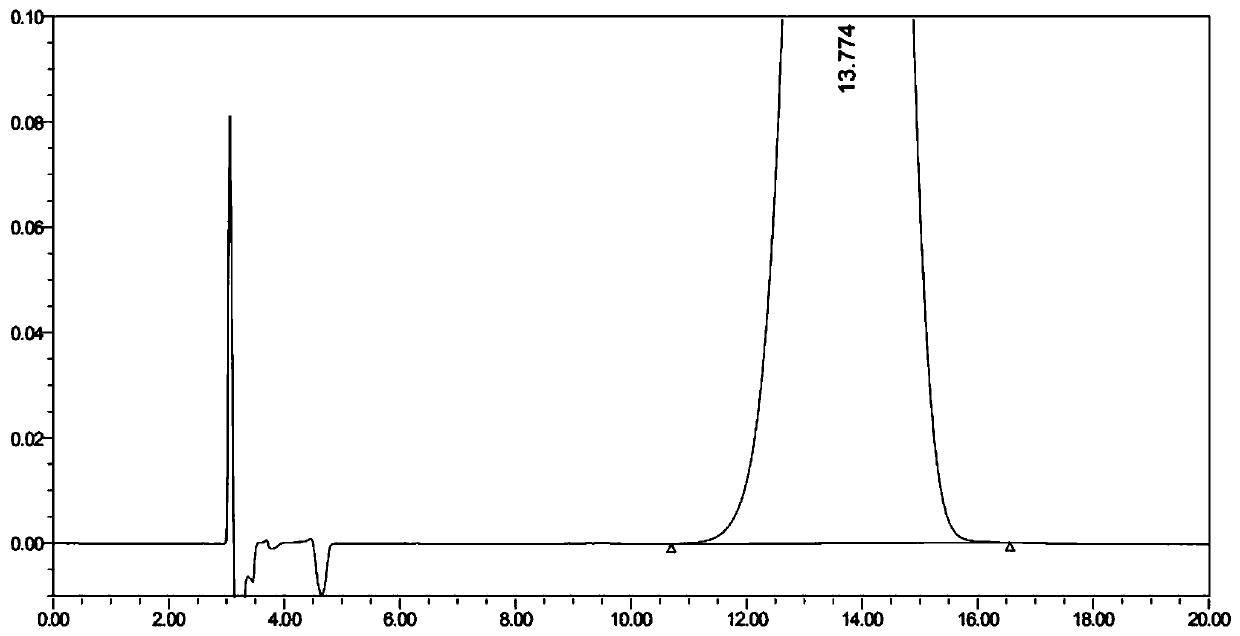
[0072] Embodiment 2 changes mobile phase ratio
[0073] By fine-tuning the proportion of the mobile phase, it was investigated whether the method is still applicable when the proportion of the mobile phase changes slightly; other conditions are the same as in Example 1 except for a slight change in the proportion of the mobile phase.
[0074] Precisely measure 10 μL of the system suitability solution and inject it into the liquid chromatograph, and record the liquid chromatogram. The change of part of the mobile phase ratio leads to inconsistency among DD-captopril, LD-captopril, and captopril. Therefore, the method should strictly control the ratio of the mobile phase, but the three can be completely separated by fine-tuning the ratio of the mobile phase. The results are shown in Table 3 below.
[0075] Table 3 Example 2 system suitability result
[0076]
Embodiment 3
[0077] Embodiment 3 changes column temperature
[0078] Investigate whether the method is still applicable when the column temperature changes slightly; except that the column temperature is adjusted to 28°C and 32°C respectively, other conditions are the same as in Example 1.
[0079] Precisely measure 10 μL of the system suitability solution and inject it into the liquid chromatograph, record the liquid chromatogram, when the column temperature is adjusted to 28°C and 32°C, DD-captopril, LD-captopril, and captopril three The degree of separation between them is greater than 1.5, which can realize complete separation. The results are shown in Table 4 below.
[0080] Table 4 Embodiment 3 system suitability result
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com