Method for detecting isomer in briviact injection by using high performance liquid chromatography
A high-performance liquid chromatography, briracetam technology, applied in the field of drug quality determination, can solve the problems of small differences in structure and polarity, difficult separation, etc., and achieve the effects of good control and accurate detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: The method for detecting the isomer in Briracetam injection by high performance liquid chromatography
[0046] The instrument used in this embodiment is an Agilent 1260 liquid chromatograph.
[0047] The chromatographic conditions adopted in this embodiment are as follows:
[0048] Chromatographic column: LUX i-Amylose-3 (4.6mm×250mm, 5μm);
[0049] Mobile phase: 20mM potassium dihydrogen phosphate aqueous solution-acetonitrile (8:2), adjust the pH value to 7.5;
[0050] Flow rate: 1.0ml / min, wavelength: 205nm, column temperature: 30°C, injection volume: 20μl.
[0051] The concrete steps of this embodiment are as follows:
[0052] (1) System suitability experiment
[0053] Take an appropriate amount of Brivaracetam reference substance and isomer impurity reference substance, dilute according to the concentration shown in Table 1 to prepare a mixed solution, and this mixed solution is used as a system suitability solution.
[0054] Table 1 Concentratio...
Embodiment 2
[0064] Embodiment 2: The influence of mobile phase flow rate on Briracetam and isomer separation degree
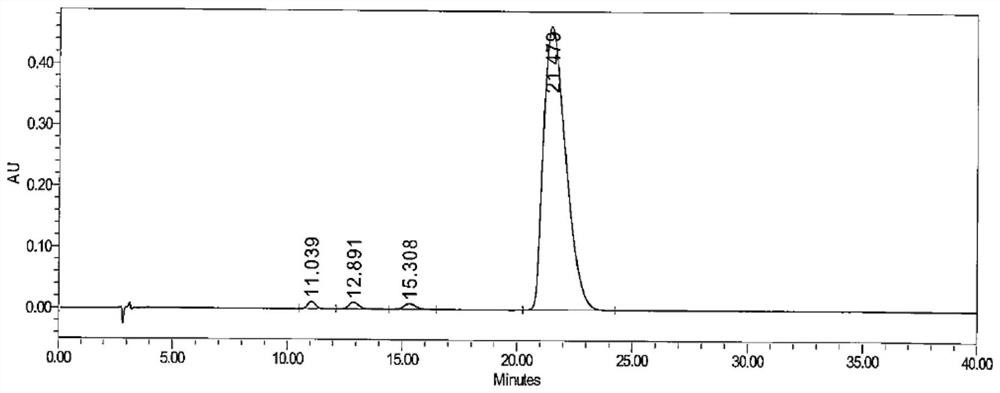
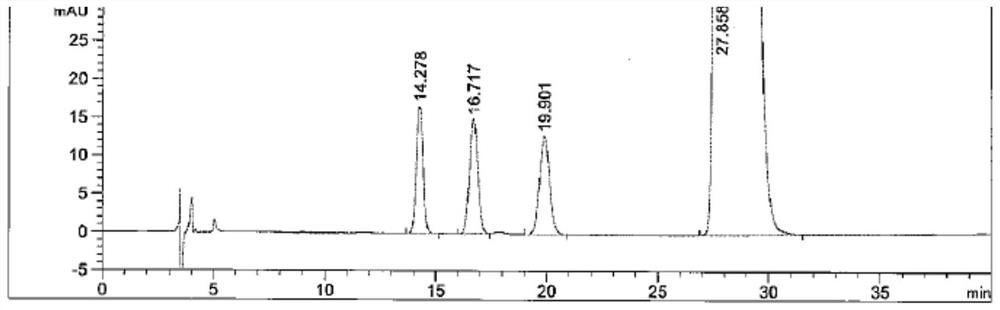
[0065] The chromatographic conditions used in this example are basically the same as those in Example 1, the only difference being that the flow rate of the mobile phase has been changed. In this embodiment, the conditions of mobile phase flow rates of 0.8ml / min, 1.0ml / min and 1.2ml / min were investigated respectively. The system suitability solution used in this example is the same as that in Example 1. Under the conditions of three mobile phase flow rates, the HPLC chromatograms of the system suitability solution are as follows: Figure 2-4 As shown, the test results are shown in Table 3.
[0066] Table 3 The effect of mobile phase flow rate on Brivaracetam and the separation of isomers
[0067]
[0068] It can be seen from the test results of this embodiment that slightly changing the flow rate of the mobile phase has little effect on the retention time and resolut...
Embodiment 3
[0069] Example 3: The influence of chromatographic column temperature on Briracetam and adjacent peak resolution
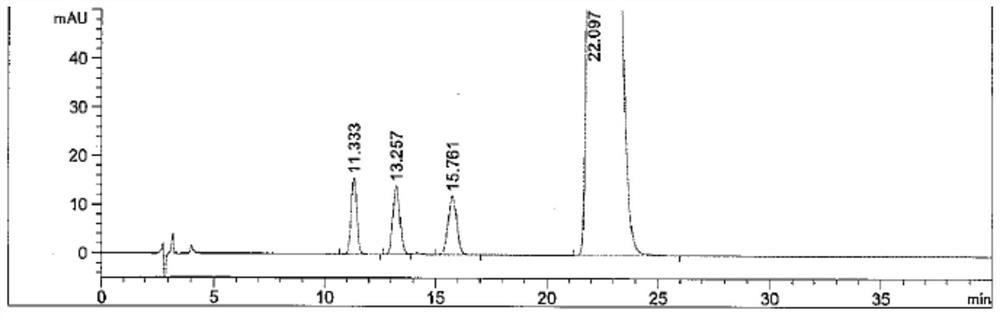
[0070] The chromatographic conditions adopted in this embodiment are basically the same as those in Example 1, the only difference being that the column temperature of the chromatographic column is changed. In this embodiment, the conditions under which the column temperature of the chromatographic column is 25° C., 30° C. and 35° C. are investigated respectively. The system suitability solution used in this example is the same as that in Example 1. Under the conditions of three mobile phase flow rates, the HPLC chromatograms of the system suitability solution are as follows: Figure 5-7 As shown, the test results are shown in Table 4.
[0071] Table 4 Effect of column temperature on the separation of Brivaracetam and its isomers
[0072]
[0073] It can be seen from the test results of this embodiment that slightly changing the column temperature of the chr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com