Detection method of chiral isomers in carfilzomib
A detection method and carfilzomib technology, applied in the field of medicine, can solve problems such as hidden safety hazards of patients, affecting the quality of carfilzomib, affecting the pharmacological activity of carfilzomib, etc., and achieving high specificity, separation and durability. Good results with high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
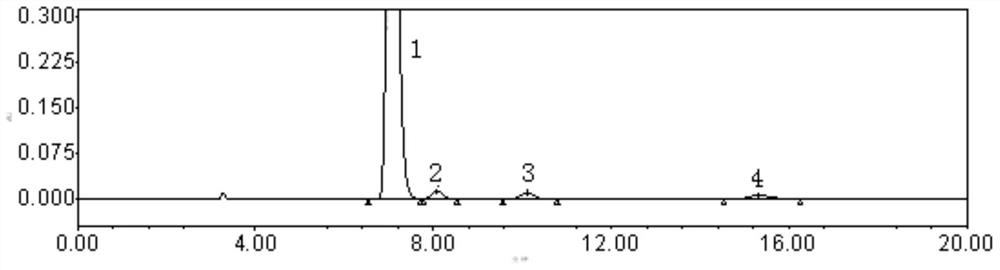
[0039] Embodiment 1 system suitability test
[0040] Instrument and chromatographic conditions:
[0041] High performance liquid chromatography Waters e2695 type (including e2489 detector and Empower chromatography workstation)
[0042] Chromatographic column: cellulose-tris(4-chloro-3-methylphenylcarbamate) bonded silica gel as filler (CHIRALCELOX-H, 4.6mm×250mm, 5μm)
[0043] Mobile phase: n-hexane-isopropanol-ethanol (89:5:6).
[0044] Flow rate: 1.0ml / min.
[0045] Detection wavelength: 220nm.
[0046] Column temperature: 40°C.
[0047] Running time: 20 minutes.
[0048] Diluent: mobile phase
[0049] Experimental steps:
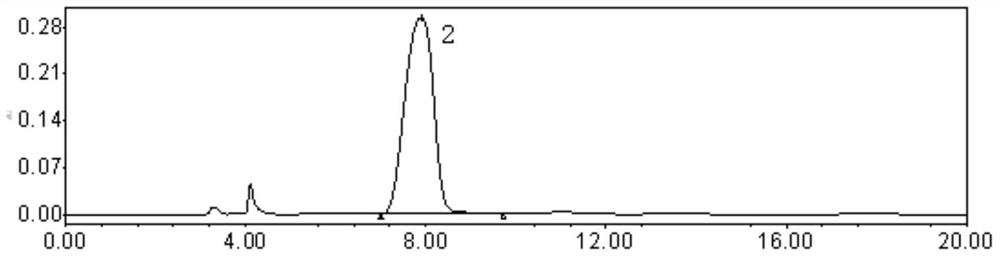
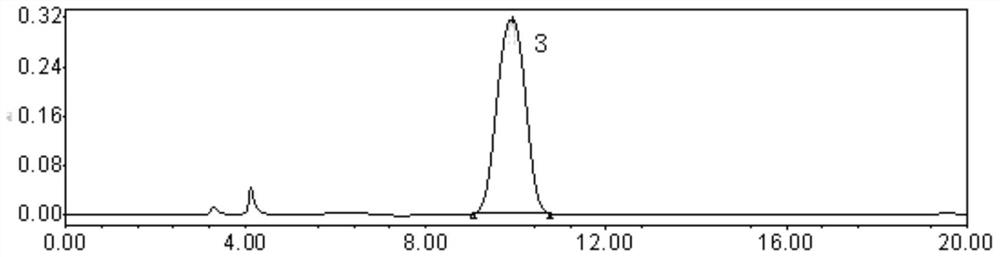
[0050] Weigh an appropriate amount of carfilzomib, enantiomer, diastereoisomer F, and diastereoisomer G reference substance, dissolve and dilute with diluent to make each 1ml containing enantiomer and a solution of 0.01 mg of each diastereoisomer and 1.0 mg of carfilzomib, shake well, filter, and use it as a system suitability solution. In additio...
Embodiment 2
[0056] Embodiment 2 The influence of mobile phase ratio on separation effect
[0057] Instrument and chromatographic conditions:
[0058] Only adjust the ratio of mobile phase n-hexane-isopropanol-ethanol (see Table 2 for details), and refer to Example 1 for other conditions.
[0059] Experimental steps:
[0060] Under the conditions of different mobile phase n-hexane-isopropanol-ethanol ratios (please see Table 2 for details), the system suitability solution was analyzed by high performance liquid chromatography respectively, and the influence of the mobile phase ratio on the separation effect of each component was investigated. The results See Table 2 for details.
[0061] Table 2 The influence of mobile phase ratio on separation effect
[0062]
[0063]
[0064] The results show that as the polarity of the mobile phase changes within the above range, the resolution and tailing factor do not change much, the column efficiency increases, and the overall separation ef...
Embodiment 3
[0065] The influence of embodiment 3 flow velocity on separation effect
[0066] Instrument and chromatographic conditions:
[0067] Only adjust the mobile phase flow rate (please refer to Table 3 for details), and refer to Example 1 for other conditions.
[0068] Experimental steps:
[0069] Under the conditions of different flow rates (see Table 3 for details), HPLC analysis was performed on the system suitability solution respectively, and the influence of mobile phase flow rate on the separation effect of each component was investigated. The results are shown in Table 3.
[0070] Table 3 The influence of flow rate on separation effect
[0071]
[0072] The results showed that in the flow rate range of 0.9-1.1ml / min, the column efficiency decreased, the tailing factor changed little, the resolution changed little, and the overall separation effect was still good, which indicated that the method had good durability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com