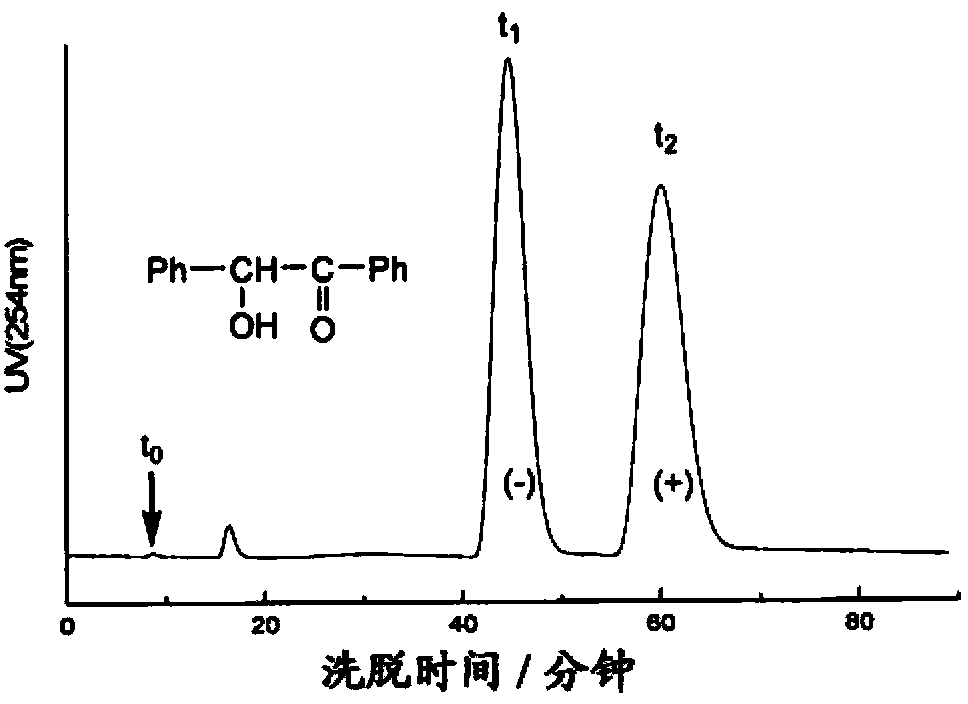
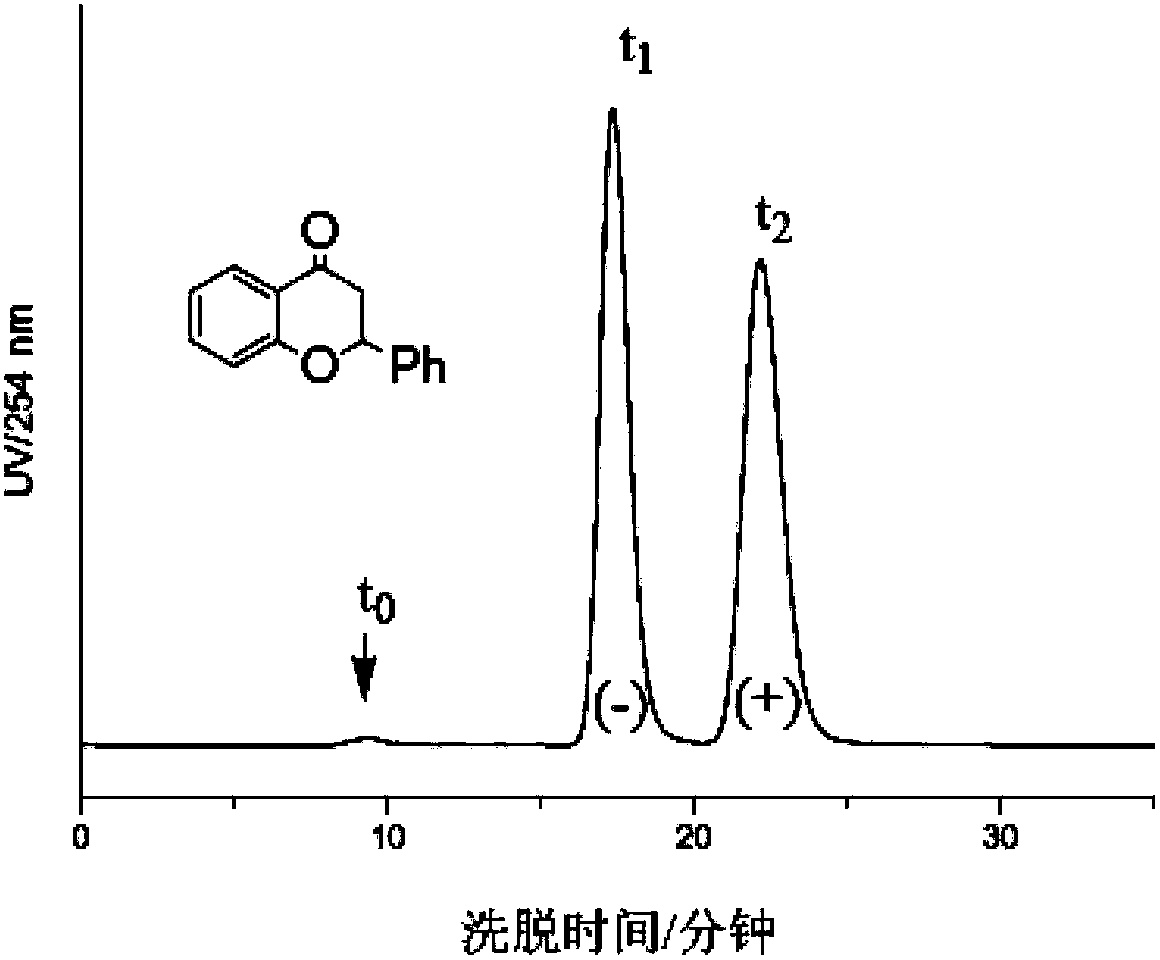
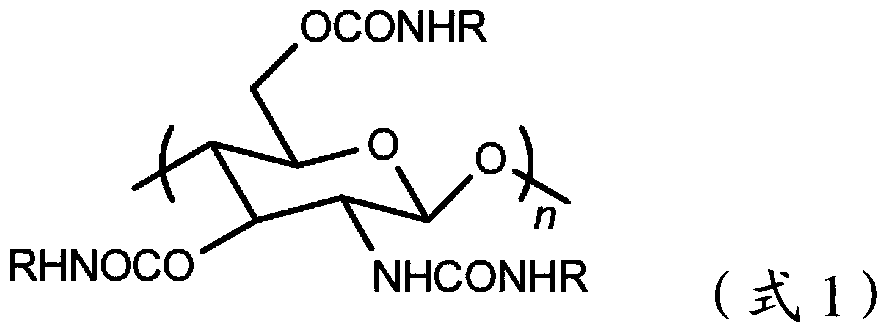
Chitosan carbanilate-carbamido derivative preparation method
A technology of phenyl carbamate and phenyl isocyanate, which is applied in the field of synthesis of chitosan derivatives, can solve problems such as poor solubility, and achieve the effects of improving solubility, good chiral recognition ability, and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Specifically, the preparation method of the present invention may comprise the following steps:
[0053] (1) add reaction solvent in chitosan raw material and carry out swelling,
[0054] (2) adding the phenyl isocyanate or phenyl isocyanate with side chains, reacting at a reaction temperature of 40-150°C, and a reaction time of 0.5-24 hours, and
[0055] (3) The reaction product is dropped into the isolation solvent to form chitosan derivatives to precipitate out.
[0056] For example, the preparation method of the present invention can be carried out as follows: under vacuum high temperature, stir the chitosan raw material of complete deacetylation for 4-6 hours, add anhydrous dimethyl sulfoxide to swell chitosan under the protection of inert gas 24- For 48 hours, add anhydrous lithium chloride at room temperature for 4-12 hours, and add phenylisocyanate with different side chains to react for 1-24 hours under high-temperature inactive gas. Afterwards, the chitosan ...
Embodiment 1
[0067] The synthetic steps of chitosan 4-methoxyphenyl carbamate-ureido derivative (a):
[0068] 1) At 80°C, 0.20g of chitosan (molecular weight: 50,000-300,000) is completely deacetylated, stirred and vacuum-dried for 4 hours.
[0069] 2) Under nitrogen protection, add 8 mL of anhydrous dimethyl sulfoxide (DMSO), and swell for 48 h.
[0070] 3) At 80°C, under nitrogen protection, add 1.60 mL of 4-methoxyphenyl isocyanate (the molar mass is 3.0 times that of hydroxyl and amino groups), and react for 24 hours.
[0071] 4) Add the above solution dropwise into 160 mL of methanol solvent with a dropper, and precipitate chitosan derivatives.
[0072] 5) Vacuum drying at 60° C. for 48 hours to obtain chitosan 4-methoxyphenylcarbamate-ureido derivatives with a yield of 63.16%.
Embodiment 2
[0074] The synthetic steps of chitosan 4-ethylphenyl carbamate-ureido derivative (b):
[0075] 1) At 80°C, 0.20g of chitosan (molecular weight: 50,000-300,000) is completely deacetylated, stirred and vacuum-dried for 4 hours.
[0076] 2) Under nitrogen protection, add 8 mL of anhydrous dimethyl sulfoxide (DMSO), and swell for 48 hours.
[0077] 3) At room temperature, add 0.40g of lithium chloride and stir for 12h.
[0078] 4) At 80°C, under nitrogen protection, add 1.06 mL of 4-ethylphenyl isocyanate (the molar mass is 2.0 times that of the hydroxyl and amino groups), and react for 1 h.
[0079] 5) Add the above solution dropwise into 160mL of methanol solvent with a dropper, and precipitate the chitosan derivative precipitate.
[0080] 6) Vacuum drying at 60°C for 48 hours, the yield of chitosan was 73.20% chitosan-4-ethylphenylcarbamate-ureido derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com