Eribulin intermediate and synthesis method and application thereof
A compound and a selected technology, which are applied in the field of intermediates of Eribulin and its synthesis, can solve the problems of long synthetic route, difficult control of optical purity of starting materials, complicated purification of intermediates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0182] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0183] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
Embodiment 1
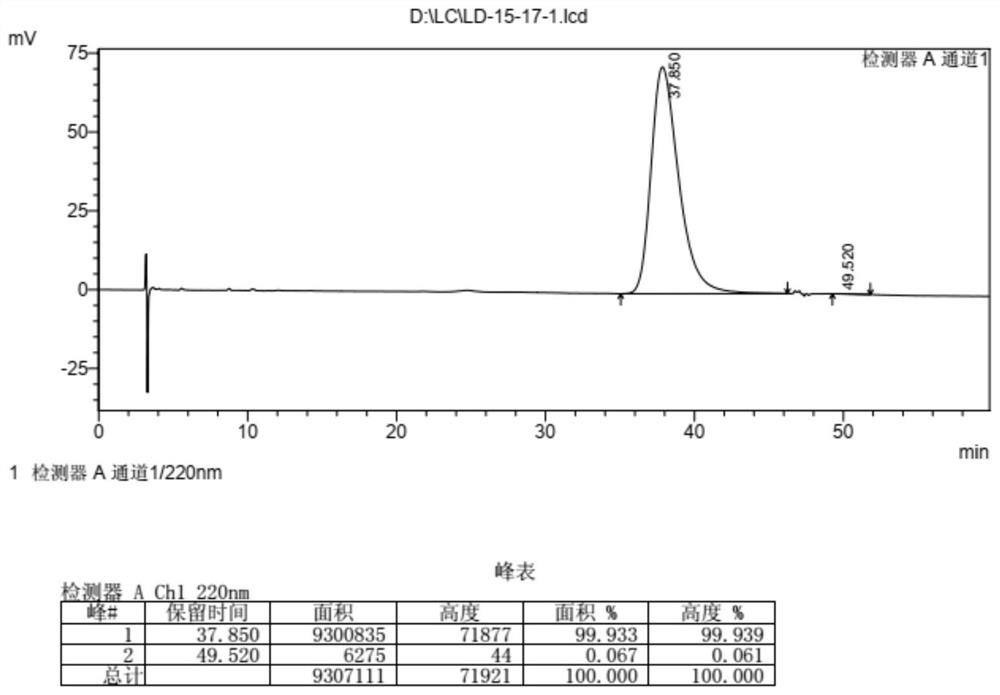
[0184] The synthesis of embodiment 1 compound 9
[0185] 1.1 Synthesis of Compound 4
[0186]
[0187] Dissolve deoxyribose 1 (100g) in water (400mL), lower the temperature to about 5°C, slowly add liquid bromine (200g) dropwise over 3 hours, and raise the temperature to 30°C for 24h after the drop (the raw material disappears as detected by spotting). Post-processing: 1) Add ethyl acetate (100mL×2) for extraction, the organic phase is in the lower layer, add ethyl acetate (50mL) for the third extraction, and the organic phase is in the upper layer; 2) Combine the organic phases and add water (50mL×2) Extract the product contained therein; 3) Combine the aqueous phases, neutralize bromine (with a small amount) with saturated sodium thiosulfate, then cool down to 0°C, adjust the pH of the solution to about 3 (the amount of solid NaOH is about 61g), Diatomaceous earth filtration; 4) When the filtrate is concentrated to a viscous liquid containing a small amount of solid, add...
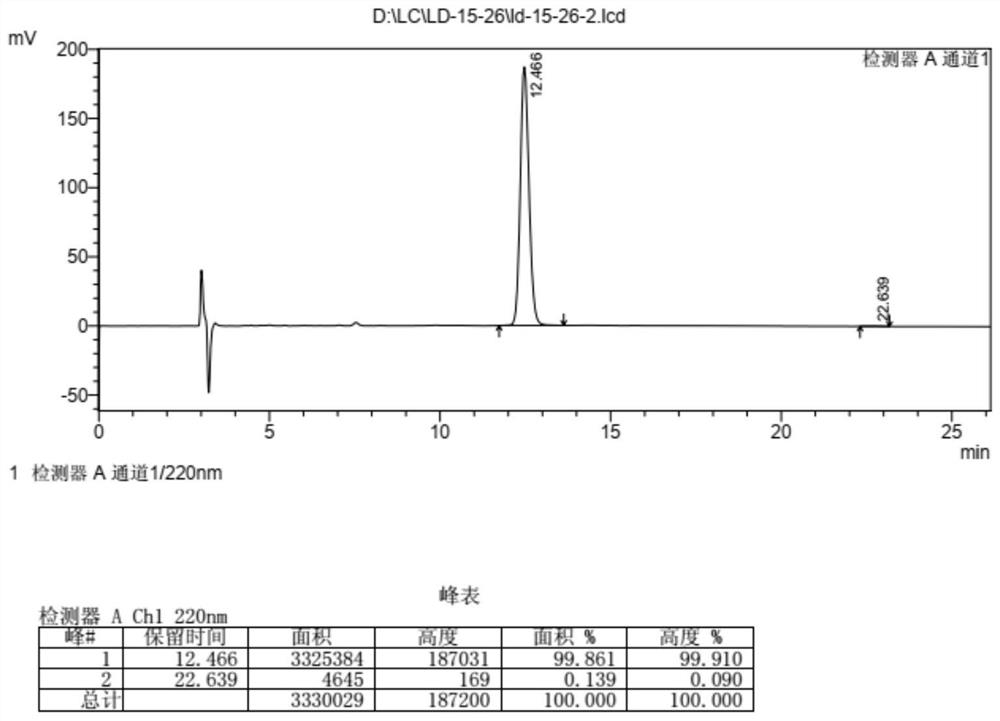
Embodiment 2
[0199] The synthesis of embodiment 2 compound 10
[0200]
[0201] Dissolve compound 9 (149.5g) in DCM (818mL), add PPTs (5.4g), add trimethyl orthoacetate (82.4mL) dropwise, react at room temperature for 1h, and evaporate the solvent to obtain the product epimer Mixture 9a.
[0202] Compound 9a obtained above was redissolved in DCM (818mL), acetyl bromide (47.9mL) was added dropwise, and reacted at room temperature for 0.5h after dropping, and the solvent was evaporated to obtain a mixture of compounds 9b-1 and 9b-2.
[0203] The above mixture of compounds 9b-1 and 9b-2 was dissolved in anhydrous methanol (1500 mL), potassium carbonate (119 g) was added, and reacted at room temperature for 8 h. Post-processing: adding saturated ammonium chloride solution, extracting with DCM, drying, filtering, concentrating, and silica gel column chromatography to obtain 123 g of the refined compound 10 with a yield of 87%. 1 H NMR (400MHz, CDCl 3 )δ7.38–7.25(m,7H),6.87(d,J=13.2Hz,2H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com