Optical active compound of 1-(3-benzoyloxy-propyl)-5-(2-(1-phenyl ethyl amine) propyl-7-cyano indoline as well as preparation method and application thereof
A technology of optical activity and compounds, applied in the direction of organic chemistry, can solve the problems that are not suitable for industrial production, and achieve the effect of easy industrial production, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: 5-((R)-2-((R)-1-phenylethylamine)propyl)-1-(3-benzoyloxypropyl)-7-cyanindole Preparation of morphine
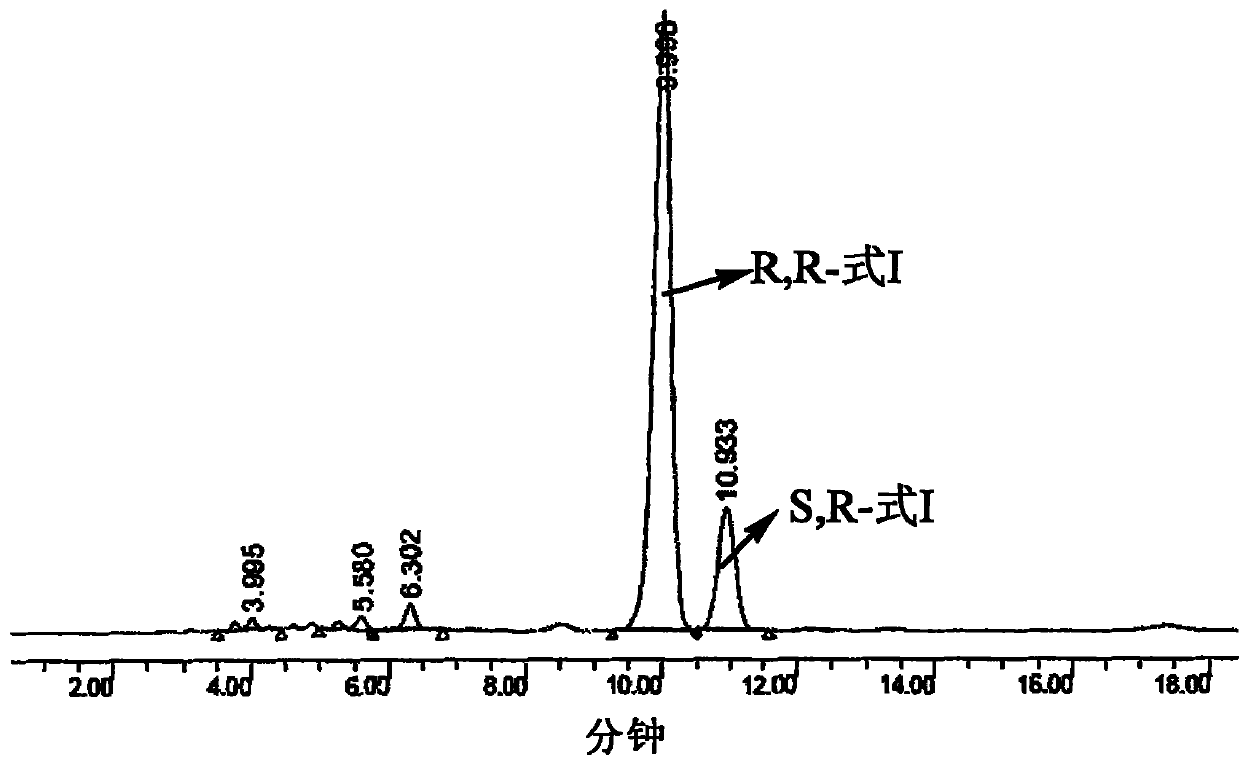
[0064] 39.11g of the compound represented by formula (II) was dissolved in dry 300mlTHF, 17g (0.140mol) of R-(+)-α-phenethylamine was added, and 8.4g (0.140mol) of AcOH, 0.5g of PtO 2 , H 2 , stirred at 60°C for 18h at 2.5 atmospheric pressure, filtered off PtO 2 , concentrated under reduced pressure. The diastereomer ratio was determined to be a 5:1 mixture by HPLC (see figure 1 ).
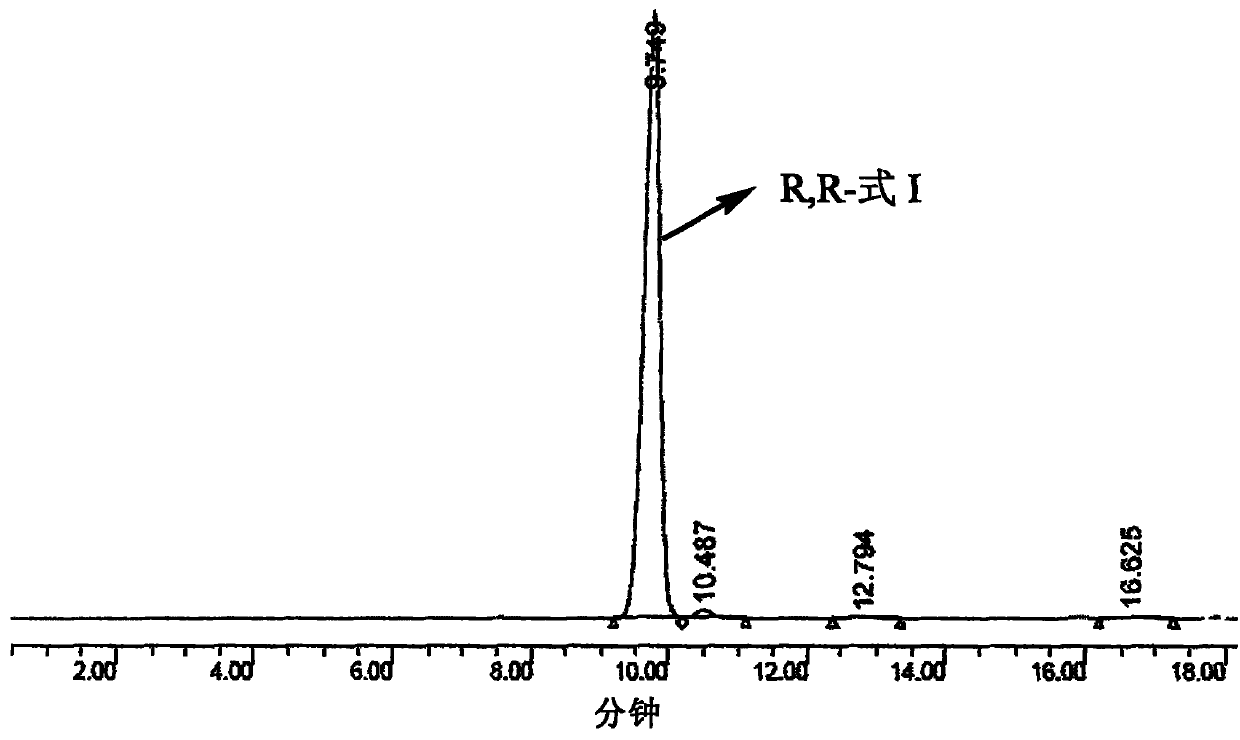
[0065] Treatment: alkali adjustment to pH 9-10, extraction with ethyl acetate, Na 2 SO 4 Dry, filter and concentrate. Salt formation: 500ml ethyl acetate, 30ml EtOH / HCl, add dropwise at 25°C, crystallize, ice-water bath, stir overnight, filter, dry, recrystallize from ethanol to obtain a single isomer, diastereomer excess 98 % (98% de value) (see figure 2 ).
[0066] Optical rotation value: [α] 20 D =+45.6, c=0.5, MeOH
[0067] Mass spectrum: m / e 468.18 (87%), 469...
Embodiment 2
[0074] Embodiment 2: the preparation of single isomer compound (IV) hydrochloride
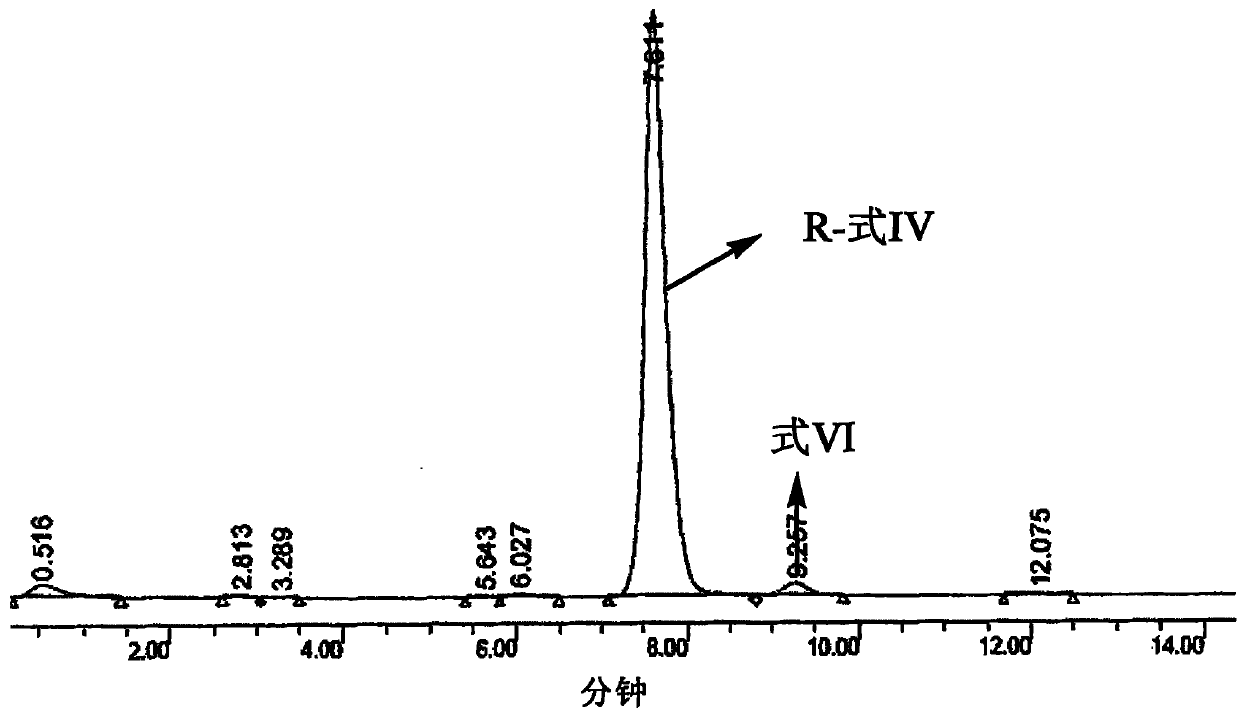
[0075] The solid that embodiment 1 obtains is placed in 50ml methanol, adds Pd / C (4.84g, 47.91mmol), feeds into H then 2 , reacted for 28 hours. Pd / C was filtered off and the filtrate was concentrated. Acetone was heated to reflux to dissolve, and cooled naturally to obtain a white solid. The reaction solution was determined by HPLC to have a purity of 94%, and very few impurity compounds such as formula (VI). Such as formula (IV) compound and formula (VI) compound ratio is 93.73: 2.38 (see image 3 )
[0076] Optical rotation value: [α] 20 D =-5.4c=1, MeOH
[0077] Mass spectrum: m / e 364.17 (87%), 365.18 (13%)
[0078] Elemental Analysis: Calculated: C 66.07, H6.56, N10.51
[0079] Measured values: C 65.87, H 6.66, N 10.54
Embodiment 3
[0081] Dissolve 1 g of the compound represented by formula (II) in 30 ml of THF, add 0.5 g of R-(+)-α-phenylethylamine, 0.05 g of Raney Ni, under hydrogen atmosphere, 60-70 ° C, 10 atm, stir for 25 h, filter off the insoluble matter, The solvent was removed under reduced pressure, alkalized, extracted with ethyl acetate, Na 2 SO 4 Dry, filter and concentrate. See embodiment 1 for physical and chemical properties.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com