Method for preparing amlodipine
A technology of amlodipine and ground level meter, which is applied in the field of medicine and chemical industry, can solve the problems of high boiling point, easy explosion, disproportionation reaction, etc., and achieve the effects of good solvent stability, low cost and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
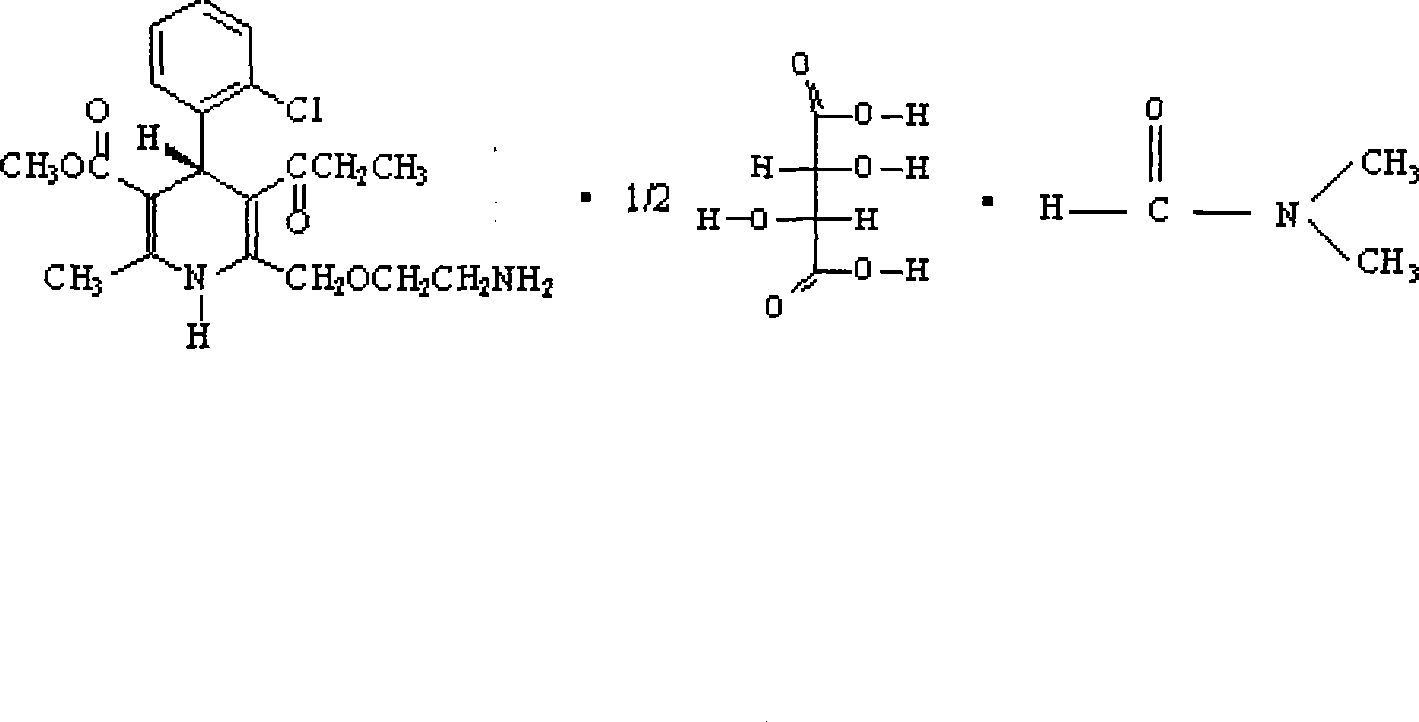
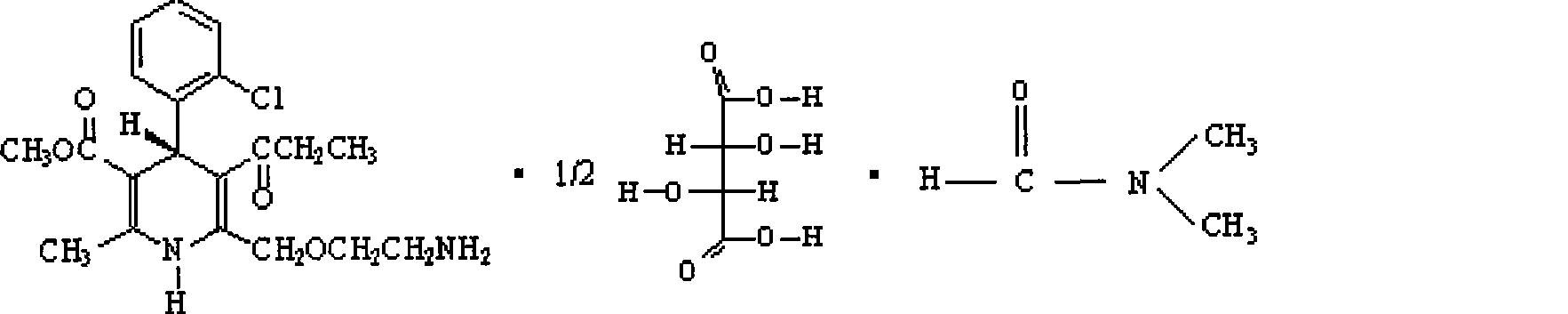
[0056] ①. Preparation of S-(-)-amlodipine-semi-L-(+)-tartrate-DMF solvate
[0057] R, S-amlodipine 50g (0.12mol), add DMF 150ml, stir to dissolve at 30°C, add dropwise a solution of L-(+)-tartaric acid 9g (0.06mol) (molar ratio 1:0.5) / DMF 240ml while stirring , after the dropwise addition, keep stirring at 30°C for 5 hours, stir at room temperature overnight, a large amount of solid precipitates, filter, wash the filter cake with a small amount of DMF, drain, add 150ml of DMF to the solid, stir for 1 hour at 30°C in a water bath, and Continue to stir overnight, filter, and wash the filter cake with a small amount of DMF, suck dry, dry, and weigh to obtain 42.2 g of S-(-)-amlodipine-half-L-(+)-tartrate-DMF solvate. Yield: 124.1% (based on S-(-)-amlodipine, the same below). Melting point: 123-128°C, ee value measurement result is 74.6%.
[0058] ②, the preparation of S-(-)-amlodipine
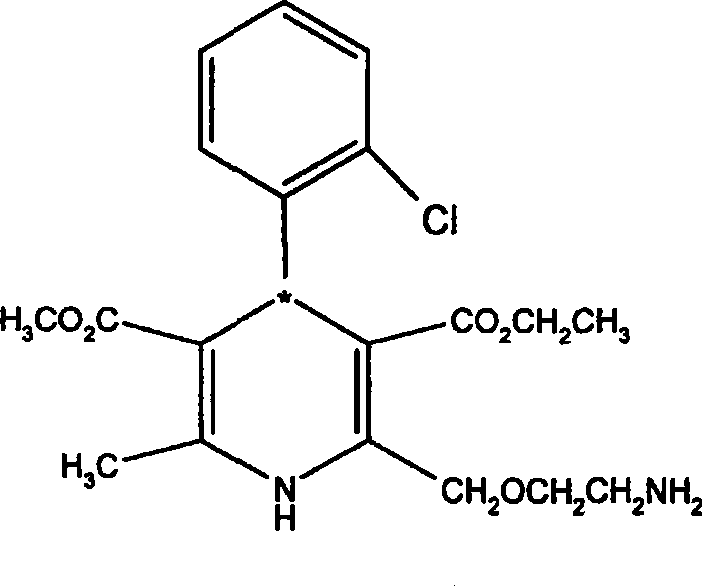
[0059] Add 100 ml of 1 mol / L sodium hydroxide aqueous solution to 10 g of S-(-)-amlodipine-...
Embodiment 2
[0062] ①. Preparation of S-(-)-amlodipine-semi-L-(+)-tartrate-DMF solvate
[0063] R, S-amlodipine 50g (0.12mol), add DMF150ml, stir to dissolve at 30°C, add L-(+)-tartaric acid 4.5g (0.03mol) (molar ratio 1:0.25) / DMF 120ml dropwise under stirring After the dropwise addition of the solution, keep stirring at 30°C for 5 hours, then stir overnight at room temperature, a large amount of solids precipitate out, filter, wash the filter cake with a small amount of DMF, drain, add 150ml of DMF to the solids, and stir for 1 hour at 30°C in a water bath. Stirring was continued at room temperature overnight, filtered, the filter cake was washed with a small amount of DMF, sucked dry, dried, and weighed to obtain 32.8 g of S-(-)-amlodipine-semi-L-(+)-tartrate-DMF solvate. Yield: 96.4%. Melting point: 125-129°C, ee value measurement result is 94.4%.
[0064] ②, the preparation of S-(-)-amlodipine
[0065] The method is the same as in Example 1, and vacuum-dried to obtain 6.7g. Yield 9...
Embodiment 3
[0067] ①. Preparation of S-(-)-amlodipine-semi-L-(+)-tartrate-DMF solvate
[0068] R, S-amlodipine 50g (0.12mol), add DMF150ml, stir to dissolve at 40°C, add L-(+)-tartaric acid 4.5g (0.03mol) (molar ratio 1:0.25) / DMF 120ml dropwise under stirring Solution, after the dropwise addition was completed, kept stirring at 40°C for 3 hours, and stirred at room temperature overnight, a large amount of solids precipitated, filtered, and the filter cake was washed with a small amount of DMF, then drained, and the solids were added to 150ml of DMF, and stirred for 1 hour at 40°C in a water bath. Stirring was continued at room temperature overnight, filtered, the filter cake was washed with a small amount of DMF, drained, dried, and weighed to obtain 32g (yield: 94.1%), melting point: 126-129°C, ee value measurement result was 95.9%.
[0069] ②, the preparation of S-(-)-amlodipine
[0070] The method is the same as in Example 1 to obtain 6.8g. (Yield 92.6%). The ee value measurement resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com