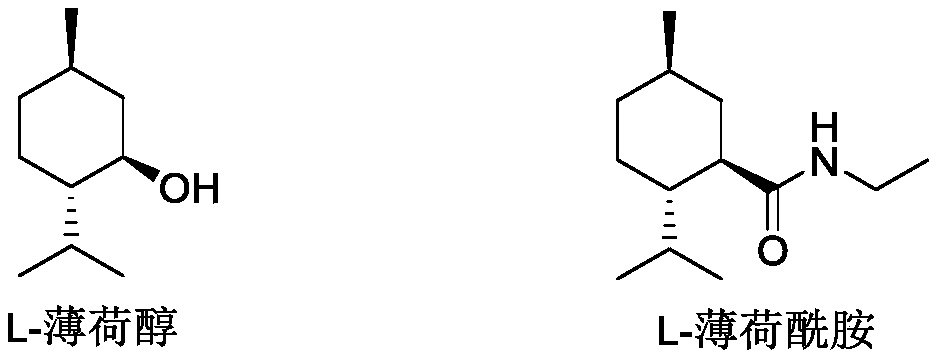
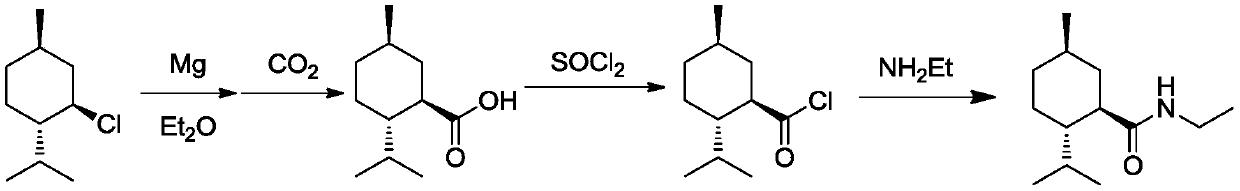
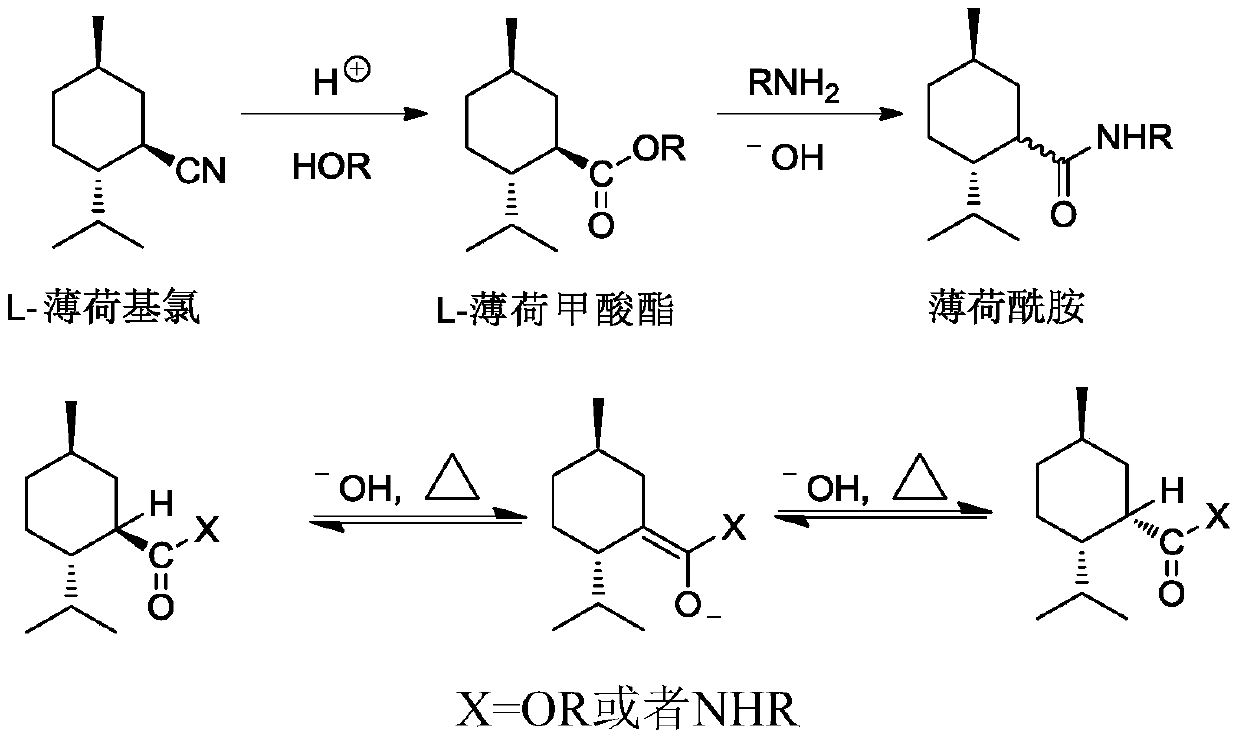
Ester ammonolysis reaction catalyst composition and preparation method of L-menthane carboxamide
A technology of menthamide and catalyst is applied in the field of ester aminolysis reaction catalyst composition, and can solve the problems of severe reaction conditions, racemization, and difficulty in industrialization, and achieve the effects of ensuring optical purity, reducing raw material costs, and reducing environmental protection costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of L-Methyl Formate
[0035] At 20°C, under the protection of N2 gas, methyl chloroformate (14.1 g, 0.15 mol) was added dropwise to the above-mentioned menthyl magnesium chloride tetrahydrofuran solution for 1 h, and then kept stirring for 0.5 h after the addition was completed. Then filter to remove the MgCl generated by the reaction 2 solid, the resulting solution was directly subjected to the next reaction.
[0036] (2) Synthesis of L-menthol amide
[0037] At 25°C, add CuBr (0.02 g, 0.15 mmol) and the ligand to the menthyl formate tetrahydrofuran solution obtained in (1) (0.05g, 0.15mmol), followed by ethylamine (6.75g, 0.15mol) for 2h. After the reaction, the reaction solution was concentrated by rectification under reduced pressure at 35°C until a small amount of solids precipitated, then dropped to 0°C within 2 hours, filtered and dried to obtain the final product, L-menthylamide, calculated as menthyl chloride. Yield 95%. The product has a melt...
Embodiment 2
[0039] (1) Synthesis of L-menthyl ethyl formate
[0040] At 25°C, under the protection of N2 gas, add ethyl chloroformate (19.4 g, 0.18 mol) dropwise to the above-mentioned menthyl magnesium chloride tetrahydrofuran solution (prepared from 0.15 mol menthyl chloride) for 1.5 hours, after the addition is completed Keep stirring for 1.0h. Then filter to remove the MgCl generated by the reaction 2 solid, the resulting solution was directly subjected to the next reaction.
[0041] (2) Synthesis of L-menthol amide
[0042] At 20°C, add CuI (0.04g, 0.225mmol) and the ligand to the menthyl formate tetrahydrofuran solution obtained in (1) (0.076g, 0.225mmol), followed by ethylamine (7.4g, 0.165mol) to react for 2h. After the reaction is over, concentrate the reaction solution by vacuum distillation at 30°C until a small amount of solid precipitates, then drop it to -5°C within 2 hours, filter and dry to obtain the final product, L-menthyl amide, calculated as menthyl chloride The...
Embodiment 3
[0044] (1) Synthesis of Isobutyl L-menthyl formate
[0045] At 30°C, under the protection of N2 gas, add propyl chloroformate (19.8 g, 0.165 mol) dropwise to the above menthyl magnesium chloride solution (prepared from 0.15 mol menthyl chloride) for 2 hours, keep stirring after the addition is completed 2.0h. Then filter to remove the MgCl generated by the reaction 2 solid, the resulting solution was directly subjected to the next reaction.
[0046] (2) Synthesis of L-menthol amide
[0047] At 30°C, add CuOAc (0.05 g, 0.3 mmol) and the ligand to the tetrahydrofuran solution of menthyl formate obtained in (1) (0.12g, 0.3mmol), followed by ethylamine (7.1g, 0.16mol) to react for 3h. After the reaction, concentrate the reaction solution at 30°C by rectification under reduced pressure until a small amount of solid precipitates out, then drop it to 5°C within 3 hours, filter and dry to obtain the final product, L-menthyl amide, calculated as menthyl chloride Yield 90%. The p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com