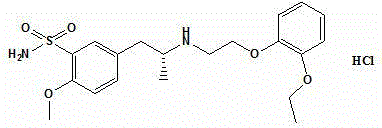
Preparation method of tamsulosin hydrochloride with high optical purity
A technology of tamsulosin hydrochloride and optical purity, applied in the fields of medical technology and chemistry, can solve the problems of time-consuming and laborious separation of enantiomers, low yield and high cost, and achieves the solution of rework treatment, good reproducibility, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride with high optical purity
[0037] Add 10 g of the crude product of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride successively to the reaction flask (Its chemical purity is 90%, e.e. value=51.3%, that is, (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxy The basic sulfonamide hydrochloride content is 68.09%, (S)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxy basic sulfonamide hydrochloride Salt content is 21.92%), ethanol 60 mL, water 30 mL, the mixture is stirred and heated to 50-60 o C dissolves and clarifies; then naturally cools down to room temperature, a small amount of white solid precipitates, and cools to 15°C in an ice-water bath o Below C, and stirred for 30 minutes; filtered, washed with 67% (volume percentage) ethanol / water, and the filter cake was discarded; the filtrate was collected, concentrated to ...
Embodiment 2
[0039] Preparation of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride with high optical purity
[0040] Add 10 g of the crude product of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride successively to the reaction flask (Chemical purity 95%, e.e. value=72.9%, that is, (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonyl The content of amide hydrochloride is 82.13%, and the content of (S)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride 12.87%), isopropanol 50 mL, water 50 mL, the mixture was stirred and heated to 50-60 o C dissolves and clarifies. Then the temperature was naturally cooled to room temperature, and a small amount of white solid was precipitated. Cool down to 15 in an ice bath o C below, and stirred for 30 minutes. Filter, wash with 50% isopropanol / water, and discard the filter cake. Collect the filtrate, concentrate to dryness under reduce...
Embodiment 3
[0042] Preparation of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride with high optical purity
[0043] Add 10 g of the crude product of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride successively to the reaction flask (e.e. value = 86.7%, which is the content of (R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride 93.35%, (S)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxysulfonamide hydrochloride content is 6.65%), Its chemical purity is 99.6%, methanol 30 mL, water 60 mL, the mixture is stirred and heated to 50-60 o C dissolves and clarifies. Then the temperature was naturally cooled to room temperature, and a small amount of white solid was precipitated. Cool down to 15 in an ice bath o C below, and stirred for 30 minutes. Filter, wash with 33% methanol / water, and discard the filter cake. The filtrate was collected, concentrated to dryness under reduced pressure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com