Method for preparing nitrendipine impurities
A technology for nitrendipine and impurities, applied in the field of nitrendipine impurity preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
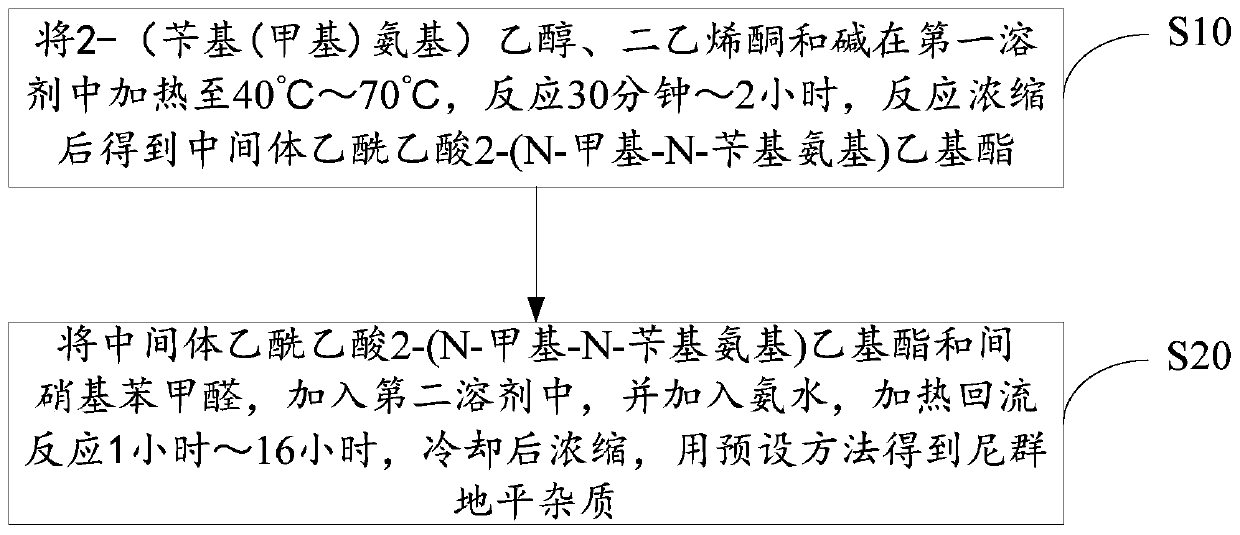
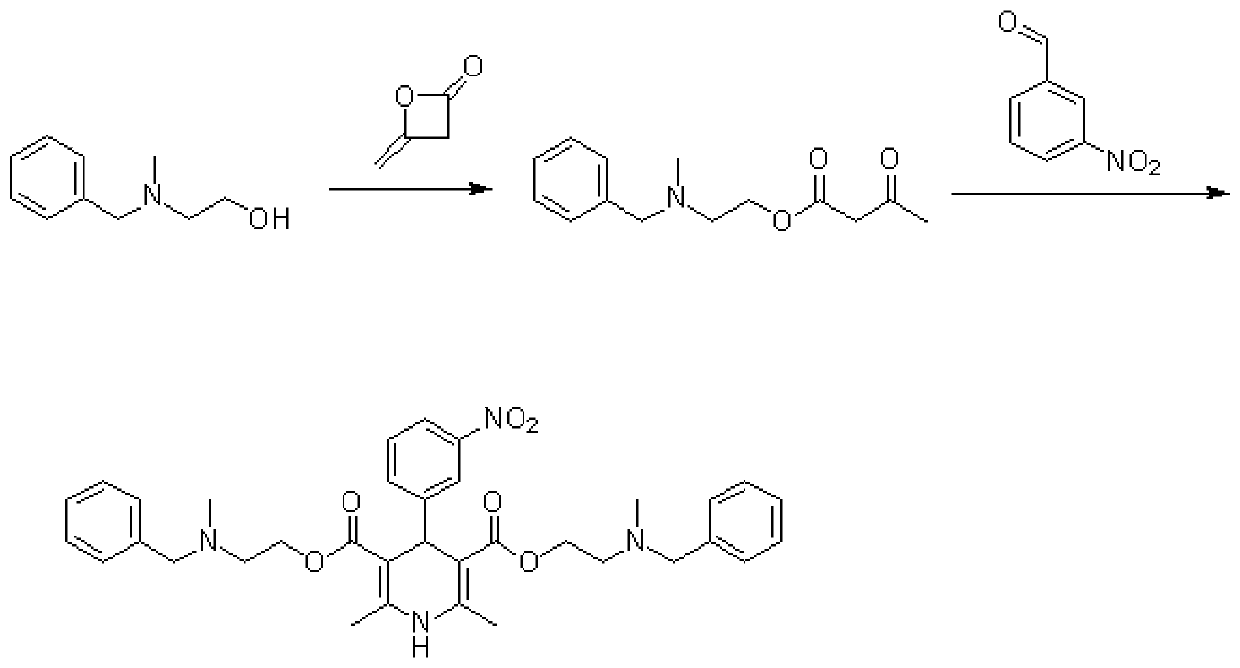
[0027] As another embodiment, it is also possible to heat 2-(benzyl(methyl)amino)ethanol, diketene and triethylamine in a mass ratio of 5:3:1 to 70°C in tetrahydrofuran as a solvent, and react for 30 Minutes, the reaction was concentrated to give the intermediate 2-(N-methyl-N-benzylamino)ethyl acetoacetate.
[0028] As another embodiment, it is also possible to heat 2-(benzyl(methyl)amino)ethanol, diketene and triethylamine in a solvent tetrahydrofuran to 55°C in a mass ratio of 5:3:1, and react for 75 Minutes, the reaction was concentrated to give the intermediate 2-(N-methyl-N-benzylamino)ethyl acetoacetate.
[0029] Wherein, the raw material quality of described 2-(benzyl (methyl) amino) ethanol, diketene and triethylamine can be 0.1 gram~100 gram, for example, and the volume of described solvent tetrahydrofuran can be 2-( 5 to 10 times that of benzyl (methyl) amino) ethanol, diketene and triethylamine.
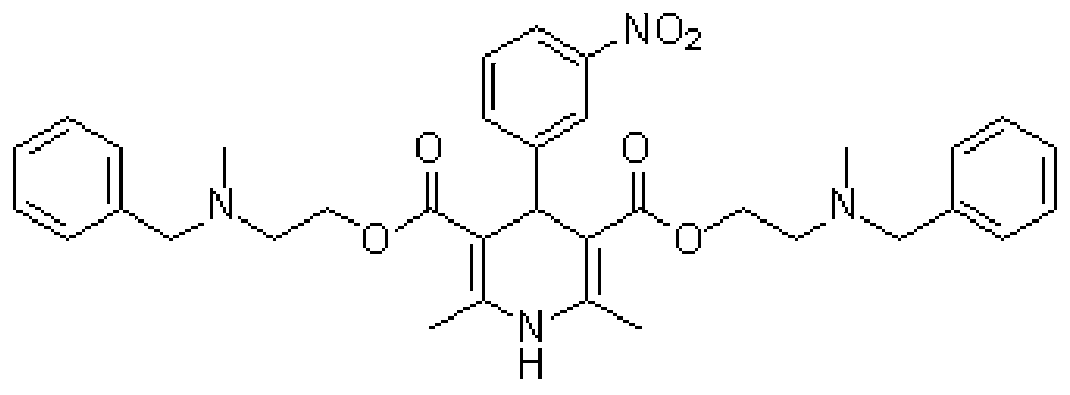
[0030] Step S20, adding the intermediate 2-(N-methyl-N-benzylamino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com