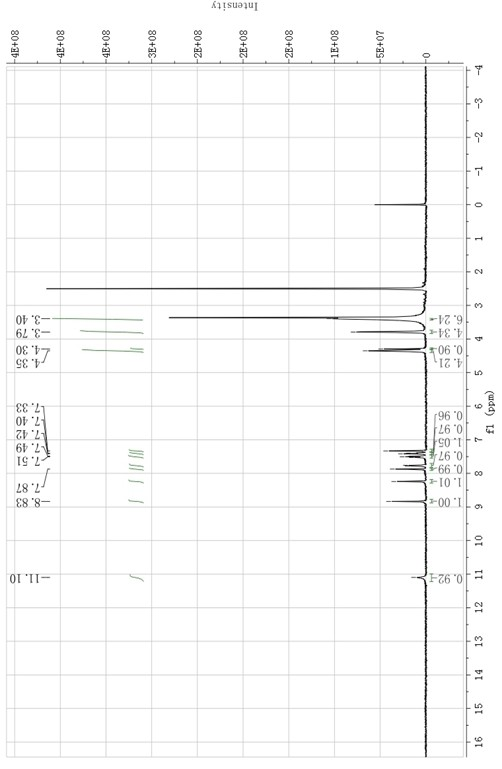
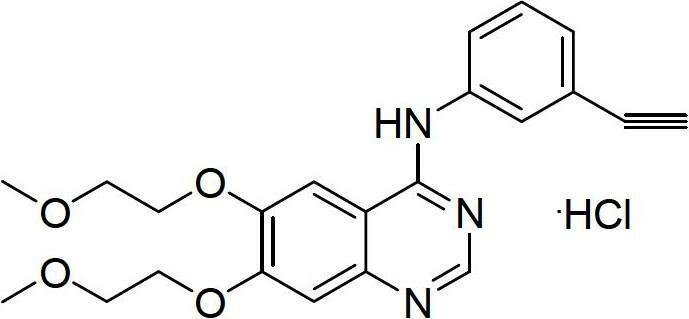
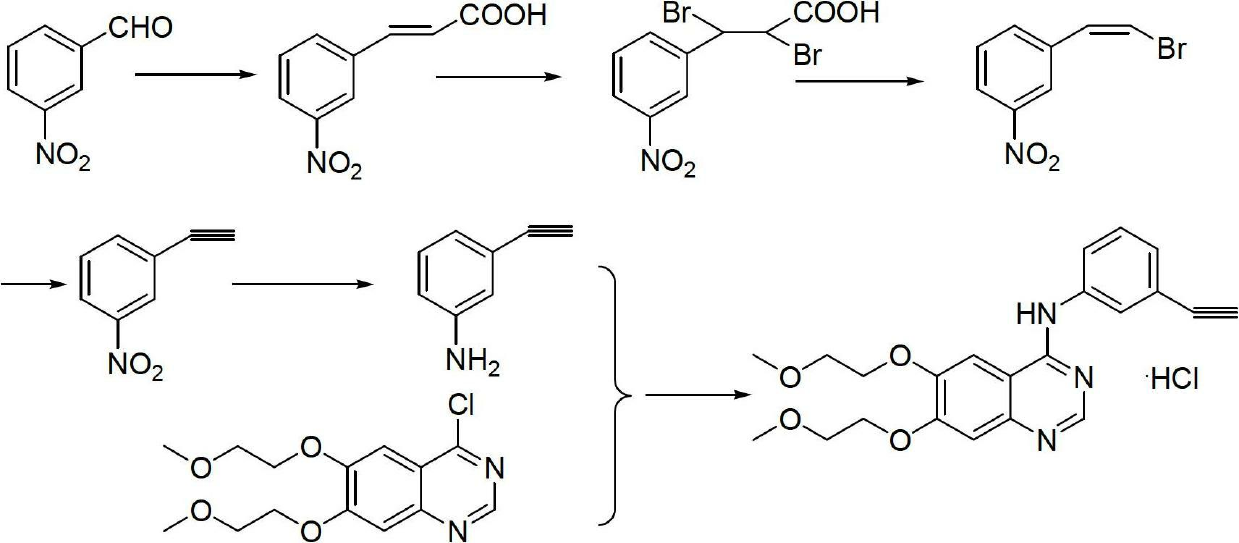
Synthesis method of erlotinib hydrochloride
A technology of erlotinib hydrochloride and its synthesis method, which is applied in the field of synthesis of erlotinib hydrochloride, can solve the problems of cumbersome operation, high raw material price and low yield, and achieve easy separation and purification, easy industrial production, and recovery high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The steps of the synthetic method of erlotinib hydrochloride are as follows:
[0031] a. React m-nitrobenzaldehyde, weak base and acetic anhydride with a molar ratio of 1:1~2:1.5~3 at 20~180°C for 5~15 hours, cool, pour into strong alkali solution, and control PH≈ 12. Extract with an organic solvent, adjust the pH of the aqueous layer to 2-3 with concentrated hydrochloric acid under ice cooling, filter with suction, wash with ice water, and dry to obtain m-nitrocinnamic acid;
[0032] b. React m-nitrocinnamic acid and bromine with a molar ratio of 1:1 to 3 in an organic solvent at 20 to 100°C for 2 to 10 hours, evaporate the solvent, and recrystallize to obtain 2,3-dibromo- 3-(3'-nitrophenyl)propanoic acid;
[0033] c. Dissolve 2,3-dibromo-3-(3'-nitrophenyl)propionic acid in dimethylformamide, cool in an ice bath to 0°C, slowly add triethylamine dropwise to 2 , The molar ratio of 3-dibromo-3-(3'-nitrophenyl)propionic acid and triethylamine is 1:1~3, then rise to room ...
Embodiment 1
[0045] (1) Preparation of m-nitrocinnamic acid
[0046] Add 30.22g (0.2mol) of m-nitrobenzaldehyde, 16.4g (0.2mol) of anhydrous sodium acetate and 30.6g (0.3mol) of acetic anhydride into a 250ml reaction flask, and heat to 180°C for 15 hours. After the reaction, cool to room temperature, pour the reaction solution into 300ml 10% sodium hydroxide aqueous solution, stir for half an hour, extract with dichloromethane (150ml×3), separate the water layer, adjust the pH value to 2-3 with concentrated hydrochloric acid, A large amount of solid was precipitated, filtered with suction, the filter cake was washed 2-3 times with ice water, and dried to obtain 33.2 g of white solid, with a yield of 86%.
[0047] (2) Preparation of 2,3-dibromo-3-(3'-nitrophenyl)propionic acid
[0048] Add 33g (0.17mol) of m-nitrocinnamic acid and 150ml of chloroform into a 250ml reaction bottle, stir and dissolve, then add 27.16g (0.17mol) of bromine, and gradually raise the temperature and reflux for 6 h...
Embodiment 2
[0058] (1) Preparation of m-nitrocinnamic acid
[0059] Add 30.22g (0.2mol) of m-nitrobenzaldehyde, 32.8g (0.4mol) of anhydrous sodium acetate and 61.25g (0.6mol) of acetic anhydride into a 250ml reaction flask, and heat to 180°C for 12 hours. After the reaction, cool to room temperature, pour the reaction solution into 300ml 10% sodium hydroxide aqueous solution, stir for half an hour, extract with dichloroethane (150ml×3), separate the water layer, and adjust the pH value to 2-3 with concentrated hydrochloric acid , a large amount of solids were precipitated, filtered with suction, the filter cake was washed 2 to 3 times with ice water, and dried to obtain 34.38 g of white solids, with a yield of 89%.
[0060] (2) Preparation of 2,3-dibromo-3-(3'-nitrophenyl)propionic acid
[0061] Add 33g (0.17mol) of m-nitrocinnamic acid and 150ml of chloroform into a 250ml reaction bottle, stir and dissolve, add bromine 81.5g (0.51mol), and gradually raise the temperature and reflux for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com