Preparation method of cardiovascular drug nilvadipine
A nilvadipine and cardiovascular technology, which is applied in the field of preparation of cardiovascular drugs, can solve the problems of difficult purification, low purity, and difficulty in obtaining pure solids, and achieves the effects of simplified procedure, improved purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
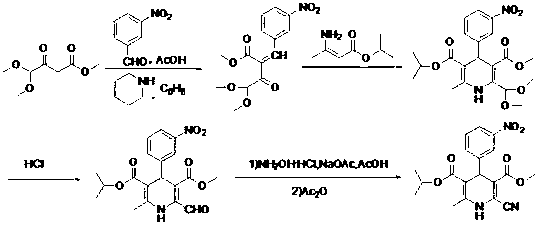
[0031] Preparation of methyl 4,4-dimethoxyacetoacetate:
[0032] Dissolve 9.6g (0.4mol) of sodium hydride with a content of 60wt%, 36.0g (0.4mol) of dimethyl carbonate, and 23.6g (0.2mol) of dimethyl acetal in 100ml of benzene to obtain a mixed solution. Reflux at 110°C for 10 h, cool the reaction solution in an ice bath, add 23 ml of glacial acetic acid dropwise, then add 62 ml of water dropwise, stir for 10 min, leave to separate layers, separate the organic layer, extract the aqueous layer with 60 ml of toluene, combine the organic layer, washed with water, dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the residue was rectified under reduced pressure to obtain 19.5g of methyl 4,4-dimethoxyacetoacetate, boiling range 65-68°C / 3mmHg, yield 55.3%.
[0033]
Embodiment 1
[0035] This embodiment includes the following steps:
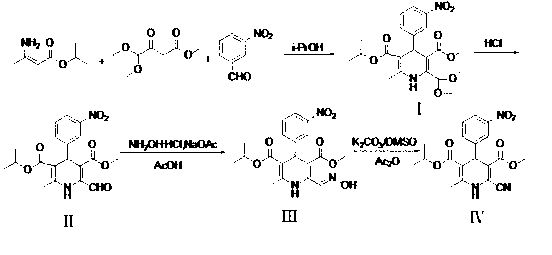
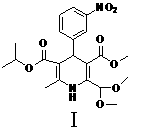
[0036] (1) 3-methoxycarbonyl-2,2-dimethoxymethyl-4-(3-nitrophenyl)-6-methyl-1,4-dihydropyridine-5-carboxyisopropyl ester (Compound Ⅰ) Preparation
[0037]5.01g (33.1mmol) m-nitrobenzaldehyde, 5.21g (36.41mmol) isopropyl 3-aminocrotonate, 6.41g (36.41mmol) methyl 4,4-dimethoxyacetoacetate and 55ml isopropyl Alcohol was mixed, heated to reflux for 10 h, the solvent was spin-dried, and the residue was recrystallized with isopropyl ether to obtain 10.4 g of white solid, yield 72.3%, m.p.110-112°C;
[0038] (2) 3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-2-formyl-1,4-dihydropyridine-5-carboxylic acid isopropyl ester (compound Ⅱ) preparation
[0039] Dissolve 7.60g (17.5mmol) of compound I in 84ml of acetone, add 10ml of 6mol / L hydrochloric acid, stir at room temperature for 5h, then add 20ml of water, stir for 20min, filter, filter cake with acetone / water (V / V=1:1 ) washing, vacuum drying to obtain 5.88g yellow powdery sol...
Embodiment 2
[0053] This embodiment includes the following steps:
[0054] (1) 3-methoxycarbonyl-2,2-dimethoxymethyl-4-(3-nitrophenyl)-6-methyl-1,4-dihydropyridine-5-carboxyisopropyl ester (Compound Ⅰ) Preparation
[0055] Mix 10.0 g (66.2 mmol) m-nitrobenzaldehyde, 10.4 g (72.8 mmol) isopropyl 3-aminocrotonate, 12.8 g (72.8 mmol) methyl 4,4-dimethoxyacetoacetate and 100 ml tetrahydrofuran , the temperature was raised to reflux for 10 h, and the solvent was removed under reduced pressure to obtain 15.0 g of a light yellow oil, which was directly used in the next step without purification;
[0056] (2) 3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-2-formyl-1,4-dihydropyridine-5-carboxylic acid isopropyl ester (compound Ⅱ) preparation
[0057] Dissolve 15.0g of the oily compound obtained in step (1) in 120ml of acetone, add 20ml of 6mol / L hydrochloric acid, stir at room temperature for 5h, add 40ml of water, stir for 20min, filter, and wash the filter cake with acetone / water (volume ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com