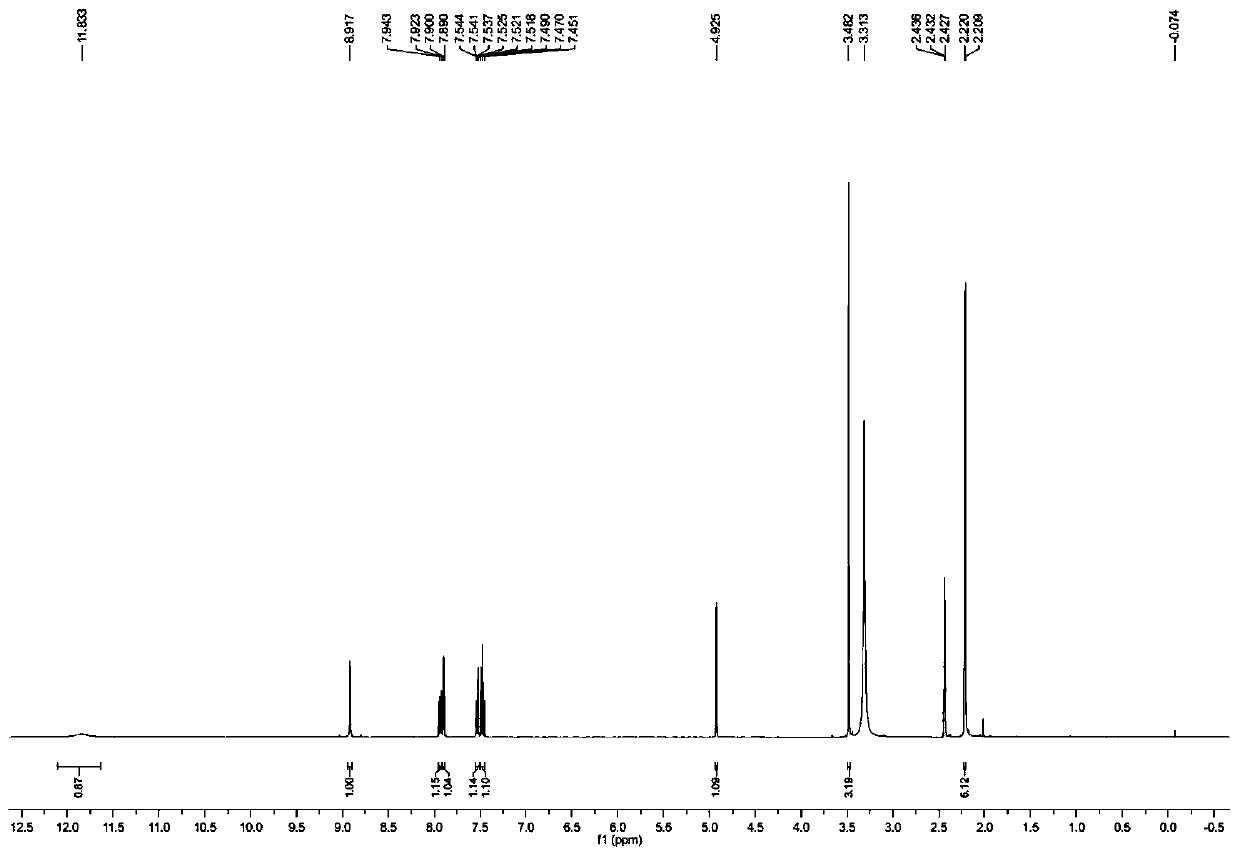
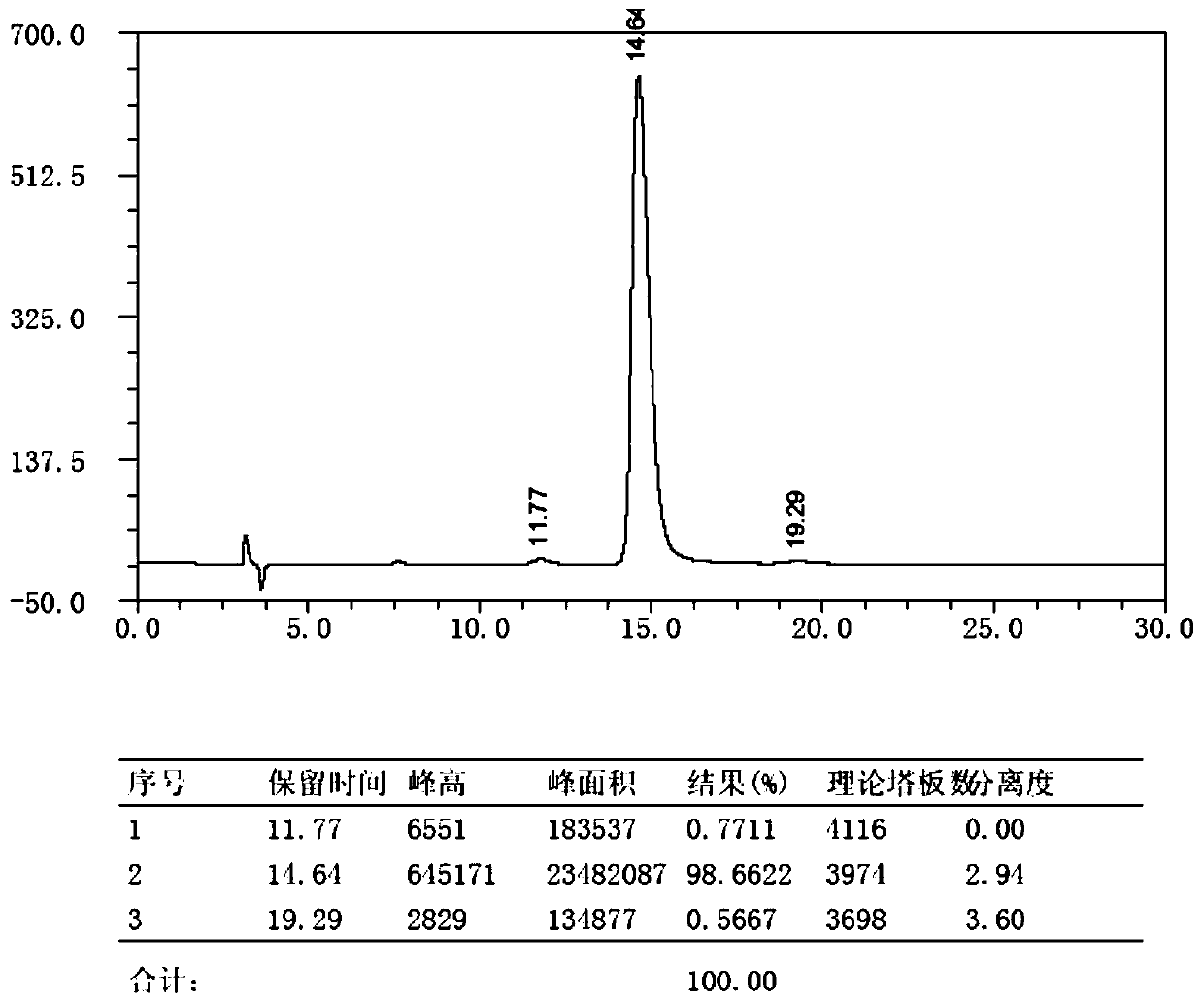
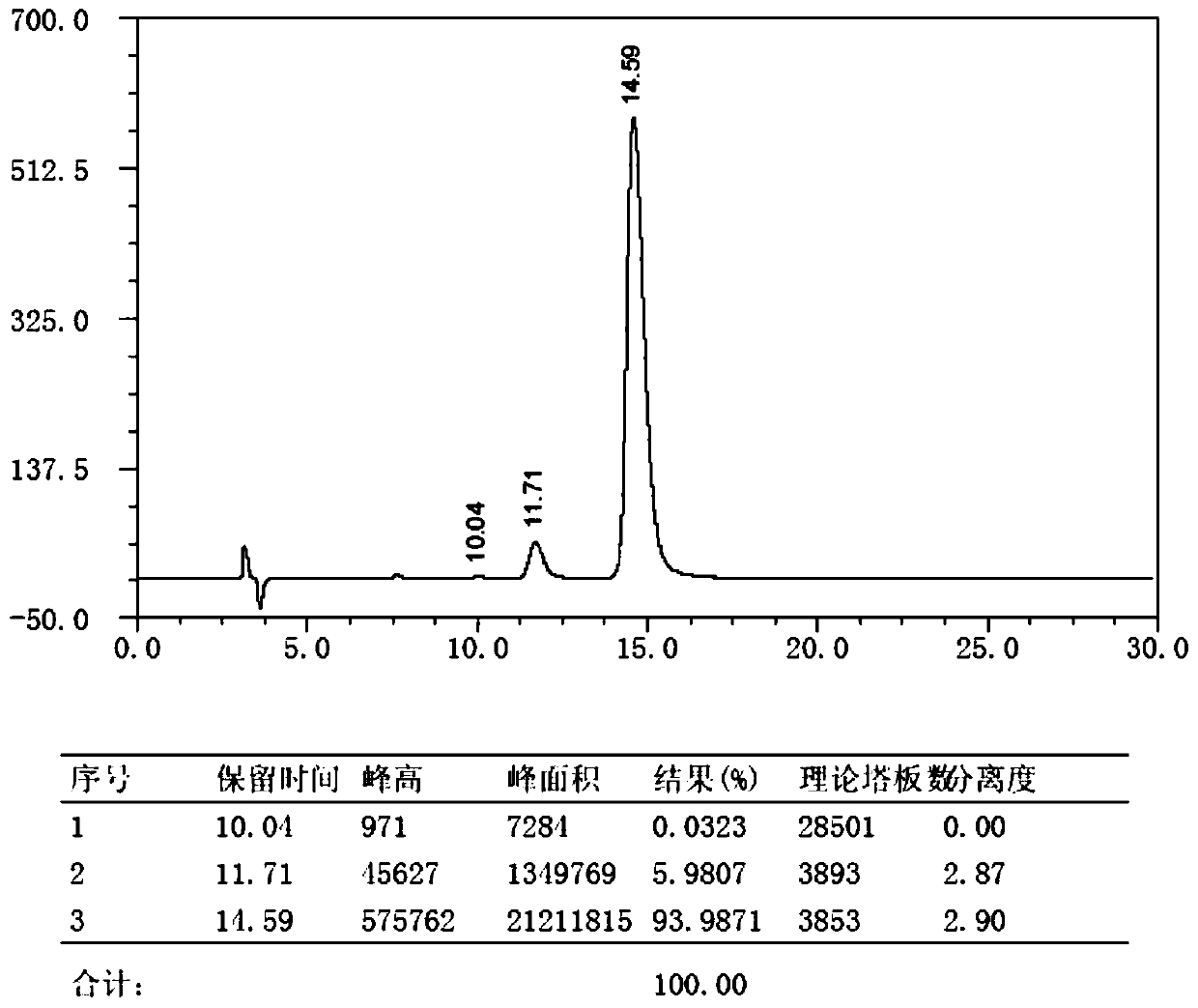
Preparation method for key intermediate of Barnidipine
A technology for barnidipine and intermediates, which is applied in the field of preparation of key intermediates of barnidipine, can solve the problems of low resolution efficiency and low yield, and achieve easy separation, high product yield, and good optical purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Barnidipine Key Intermediate (R)1,4-Dihydro-2,6-Dimethyl-4-(3-nitrophenyl)-5 Using L-Lactic Acid as Chiral Hydroxy Acid as Starting Material -Methoxycarbonyl-3-pyridinecarboxylic acid, the specific preparation steps are as follows:
[0036] Step 1: Preparation of L-isopropyl lactate (intermediate 1)
[0037] Take 225g of isopropanol, cool in an ice bath for 15min, add 3ml of concentrated sulfuric acid dropwise, add 106g of L-lactic acid and 500ml of toluene, conduct azeotropic dehydration reaction at 140°C for 12h, then evaporate the solvent under reduced pressure at 50°C, and add ethyl acetate to the residue 500ml of ester, 500ml of saturated sodium bicarbonate solution, stirred for 10min, separated the water layer, washed the organic layer with 500ml of saturated sodium chloride, dried over anhydrous sodium sulfate, and distilled off the solvent at 40°C under reduced pressure to obtain L - Isopropyl lactate, weighing 80g, yield 60.6%, directly dropped ...
Embodiment 2
[0047] Preparation of the key intermediate of barnidipine (R)1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 5-methoxycarbonyl-3-pyridinecarboxylic acid, the specific preparation steps are as follows:
[0048] Step 1: Preparation of S-isopropyl phenyllactate (intermediate 1)
[0049] Put 16.6g of S-phenyllactic acid and 300ml of isopropanol into the reaction flask in turn, add 1.7g of boric acid under stirring, stir at room temperature for 24h, evaporate the solvent under reduced pressure at 40°C, add 500ml of dichloromethane to the residue to dissolve, and then use saturated Wash with 500ml of sodium bicarbonate solution, 500ml of saturated sodium chloride, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure at 40°C to obtain S-isopropyl phenyllactate as a colorless transparent paste, weighing 18.9g, yield 91%, put directly into the next step reaction.
[0050] Step 2: Preparation of (S)-α-3-oxobutyryloxyphenylpropionate isopropyl (intermediate 2...
Embodiment 3
[0059] Preparation of the key intermediate of barnidipine (R)1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 5-methoxycarbonyl-3-pyridinecarboxylic acid, the specific preparation steps are as follows:
[0060] Step 1: Preparation of S-isopropyl mandelate (intermediate 1)
[0061] Put 15.2g of S-mandelic acid and 300ml of isopropanol into the reaction flask in turn, add 1.7g of boric acid under stirring, stir at room temperature for 24h, evaporate the solvent at 40°C under reduced pressure, add 500ml of dichloromethane to the residue to dissolve, and use saturated Wash with 500ml of sodium bicarbonate solution, 500ml of saturated sodium chloride, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure at 40°C to obtain S-isopropyl mandelate as a colorless transparent paste, weighing 18.0g, yield 93%, put directly into the next step reaction.
[0062] Step 2: Preparation of (S)-α-3-oxobutanoyloxyphenylacetic acid isopropyl ester (intermediate 2)
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com