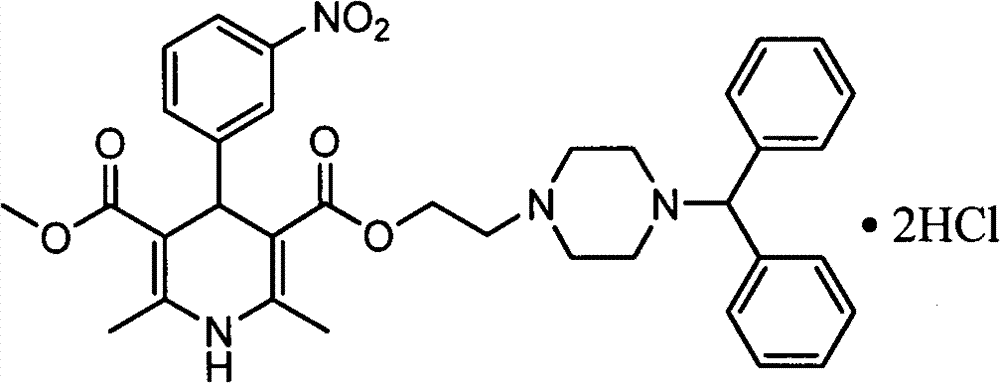
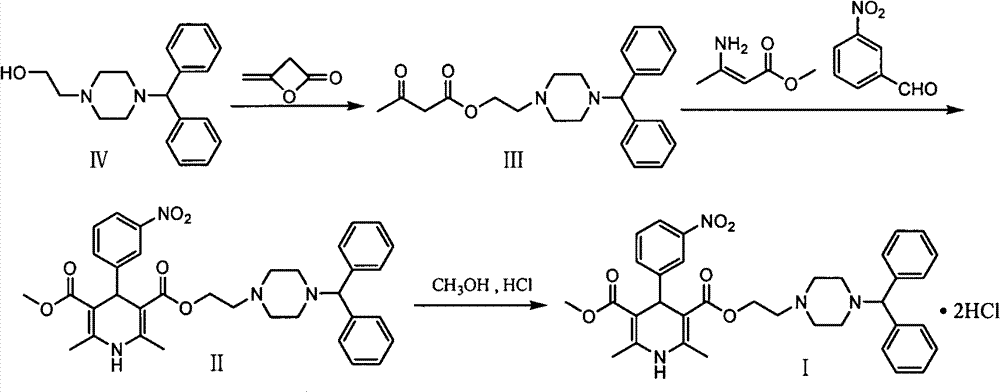
Improved method for synthesis process of manidipine hydrochloride
A manidipine hydrochloride and synthetic process technology, applied in the direction of organic chemistry, can solve the problems of low product purity, complexity and unsuitability for industrial production, and achieve the effects of reducing costs, shortening production cycle, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Add 1.22kg (4.12mol) 1-benzhydryl-4-(2-hydroxyethyl)piperazine (IV) into the reaction flask, heat to 70-75°C, add dropwise 0.43kg ( 5.12mol) of diketene, after dropping, keep stirring at 70-80°C for 2h. After the reaction was completed, 6L of ethyl acetate was added for dissolution, and the organic phase was washed with water for 3 times, dried, and concentrated to obtain oily substance 2-(4-benzhydryl-1-piperazinyl) ethyl acetoacetate (III) 1.50 kg (yield: 95.8%).
[0020] Step 2: 1.50kg (3.94mol) of the above-mentioned oil (III) and 0.45kg (3.91mol) of methyl 3-aminocrotonate, 0.59kg (3.90mo) of m-nitrobenzaldehyde, and 4.95kg of isopropanol are dissolved in In a 10L reaction flask, heat and reflux for 7 hours, distill out isopropanol, add 15L ethyl acetate to the residue to dissolve, wash with water, separate liquid, concentrate, add 3L methanol to dissolve, adjust the pH value of the system to between 1-2 with concentrated hydrochloric acid 15L ethyl aceta...
Embodiment 2
[0023] Step 1: Add 1.22kg (4.12mol) 1-benzhydryl-4-(2-hydroxyethyl)piperazine (IV) into the reaction flask, heat to 70-75°C, add dropwise 0.43kg ( 5.12mol) of diketene, after dropping, keep stirring at 70-80°C for 2h. After the reaction was completed, 8 L of methyl acetate was added for dissolution, and the organic phase was washed with water three times, dried, and concentrated to obtain 1.47 kg of oil (III) (yield: 93.9%).
[0024] Step 2: Dissolve 1.1.47kg (3.86mol) of the above-mentioned oil (III) and 0.44kg (3.82mol) of methyl 3-aminocrotonate, 0.58kg (3.84mo) of m-nitrobenzaldehyde, and 4.95kg of isopropanol In a 10L reaction flask, heat and reflux for 7 hours, distill out isopropanol, add 17L methyl acetate to the residue to dissolve, wash with water, separate liquid, concentrate, add 3L methanol to dissolve, adjust the pH value of the system to 1-2 with concentrated hydrochloric acid 17L methyl acetate to dissolve the filter cake, adjust the pH value of the system wit...
Embodiment 3
[0027] Step 1: Add 1.22kg (4.12mol) 1-benzhydryl-4-(2-hydroxyethyl)piperazine (IV) into the reaction flask, heat to 70-75°C, add dropwise 0.43kg ( 5.12mol) of diketene, after dropping, keep stirring at 70-80°C for 2h. After the reaction was completed, 6L of ethyl acetate was added for dissolution, and the organic phase was washed with water for 3 times, dried, and concentrated to obtain oily substance 2-(4-benzhydryl-1-piperazinyl) ethyl acetoacetate (III) 1.52 kg (yield: 97.1%).
[0028] Step 2: 1.50kg (3.94mol) of the above-mentioned oil (III) and 0.45kg (3.91mol) of methyl 3-aminocrotonate, 0.59kg (3.90mo) of m-nitrobenzaldehyde, and 4.95kg of isopropanol are dissolved in In a 10L reaction flask, heat and reflux for 7 hours, distill out isopropanol, add 15L ethyl acetate to the residue to dissolve, wash with water, separate liquid, concentrate, add 3L methanol to dissolve, adjust the pH value of the system to between 1-2 with concentrated hydrochloric acid 15L ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com