Preparation method of zaleplon
A technology of zaleplon and synthetic route, applied in the direction of organic chemistry, can solve the problem of high yield, achieve the effect of short route, reduce synthesis cost, and avoid the production of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
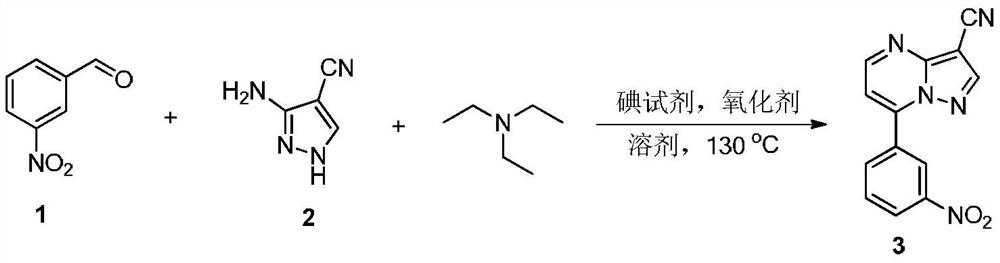
[0025] Preparation of compound 3
[0026]
[0027] Add m-nitrobenzaldehyde 1 (75.5mg, 0.5mmol), 3-amino-4-cyanopyrazole 2 (54mg, 0.5mmol), triethylamine (101mg, 1mmol), ammonium iodide in a 35mL sealed tube (72.5mg, 0.5mmol), di-tert-butyl peroxide (219mg, 1.5mmol) and toluene (2mL), then placed in an oil bath at 130°C and stirred for 10h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=3 / 1, v / v) to obtain compound 3 (114 mg, 86%) as a yellow solid product. The characterization data of this compound are as follows: 1 H NMR (400MHz, DMSO-d 6 ): δ (ppm) 8.97 (d, J = 1.6Hz, 1H), 8.95 (d, J = 4.8Hz, 1H), 8.88 (s, 1H), 8.48 (dd, J = 8.0, 2.0Hz, 2H) ,7.92(t,J=8.0Hz,1H),7.72(d,J=4.4Hz,1H); 13...
Embodiment 2
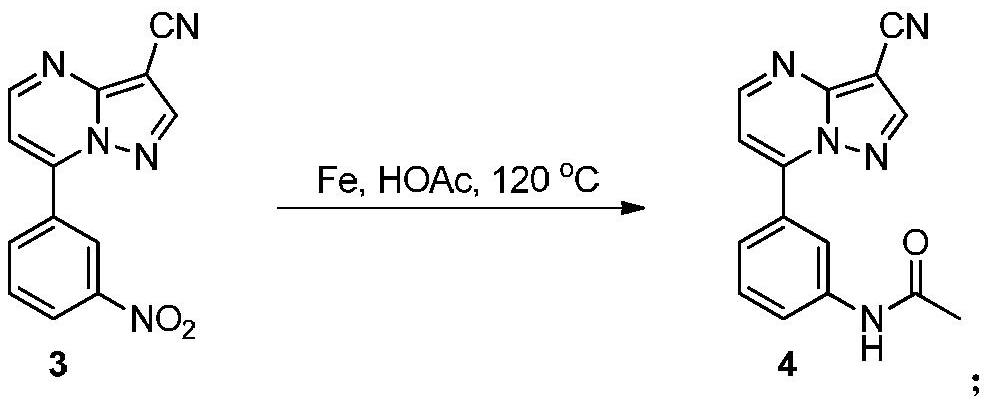
[0029] Preparation of Compound 4
[0030]
[0031] Iron powder (184.8 mg, 3.3 mmol) and glacial acetic acid (20 mL) were put into a three-necked flask, stirred and heated to reflux, acidified for 10 min, and a solution of compound 3 (265 mg, 1 mmol) in glacial acetic acid (25 mL) was added dropwise. After the dropwise addition, continue to reflux for 4h. The reaction was quenched by adding 50 mL of water, extracted with ethyl acetate (50 mL×3), and then the organic phase was washed with saturated brine and dried over anhydrous sodium sulfate. Filtered, spin-dried, and dried to give compound 4 (230 mg, 83%) as a white solid. The characterization data of this compound are as follows: 1 HNMR (400MHz, DMSO-d 6 ):δ(ppm)10.25(s,1H),8.89(d,J=4.4Hz,1H),8.86(s,1H),8.32(t,J=1.8Hz,1H),7.85–7.81(m, 1H), 7.70–7.67(m, 1H), 7.54(t, J=8.0Hz, 1H), 7.50(d, J=4.8Hz, 1H), 2.08(s, 3H); 13 C NMR (100MHz, DMSO-d 6 ):δ(ppm)168.7,153.8,151.1,147.5,147.3,139.4,129.9,129.1,124.3,122.0,119.9,113...
Embodiment 3
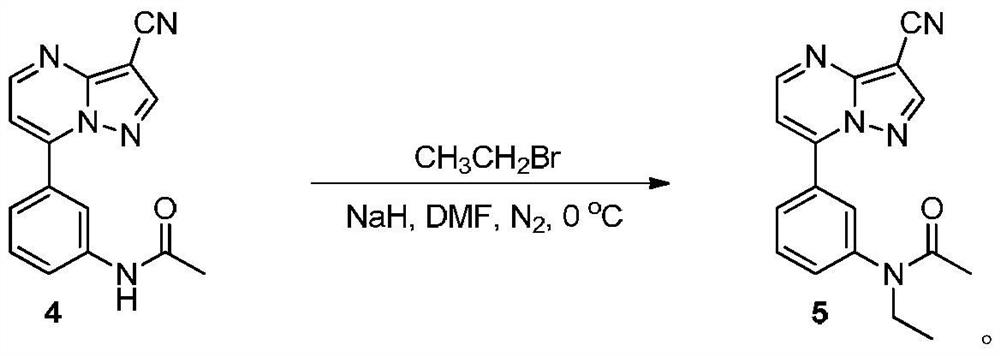
[0033] Preparation of compound 5
[0034]
[0035]Sodium hydride (58mg, 2.4mmol) and anhydrous DMF (2mL) were put into a three-necked flask filled with nitrogen, stirred in an ice-water bath, a DMF (6mL) solution of compound 4 (110.8mg, 0.4mmol) was added dropwise, and After the addition was completed, reacted for 5 minutes, added bromoethane (436 mg, 4 mmol) dropwise, and continued to stir in an ice-water bath for 30 minutes. It was extracted with ethyl acetate (50 mL×3), and the organic phase was washed with saturated brine and dried over anhydrous sodium sulfate. Filtered, spin-dried, and dried to give compound 5 (103.7 mg, 85%) as a white solid. The characterization data of this compound are as follows: 1 H NMR (400MHz, DMSO-d 6 ): δ (ppm) 8.92 (d, J = 4.4Hz, 1H), 8.87 (d, J = 0.4Hz, 1H), 8.09 (d, J = 7.6Hz, 1H), 8.04 (s, 1H), 7.71 (t, J=7.8Hz, 1H), 7.67(d, J=4.4Hz, 1H), 7.61(d, J=7.2Hz, 1H), 3.70(q, J=6.8Hz, 2H), 1.81(s ,3H),1.03(t,J=6.8Hz,3H); 13 C NMR (100MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com