Phenanthroimidazole-fluorescein pH fluorescent probe containing two hydroxyl groups and preparation method of phenanthroimidazole-fluorescein pH fluorescent probe
A technology of phenanthroimidazole and fluorescent probe, which is applied in the field of phenanthroimidazole-fluorescein pH fluorescent probe and its preparation, can solve the problem of single structure, few modification sites of pH fluorescent probe, no clear response site, etc. problem, to achieve the effect of stable structure, high sensitivity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
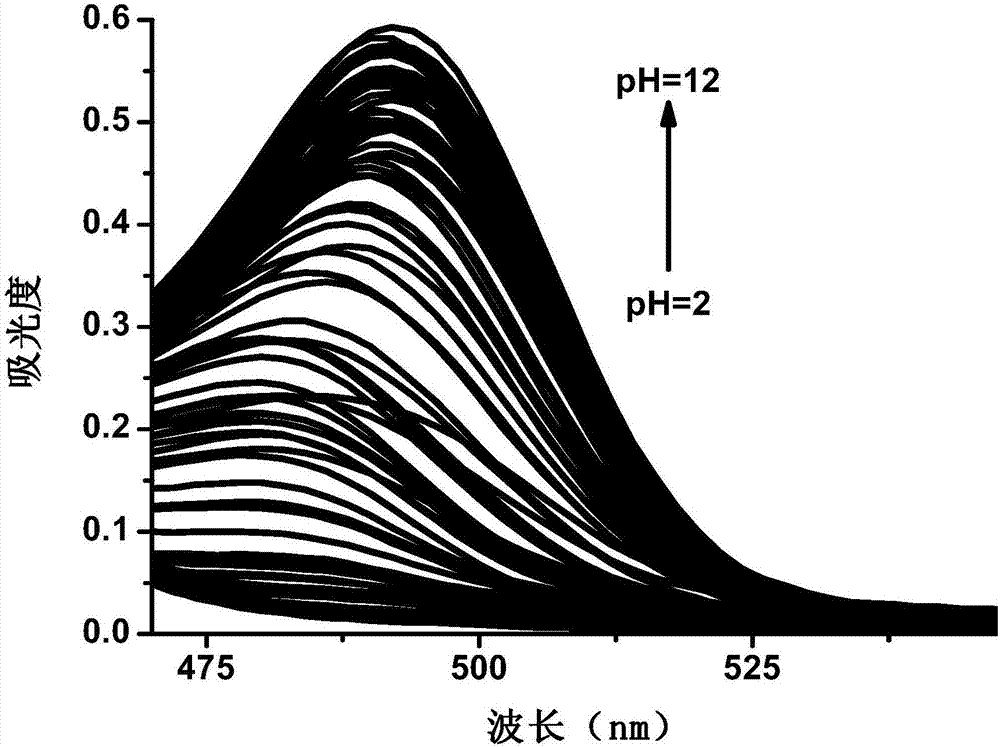
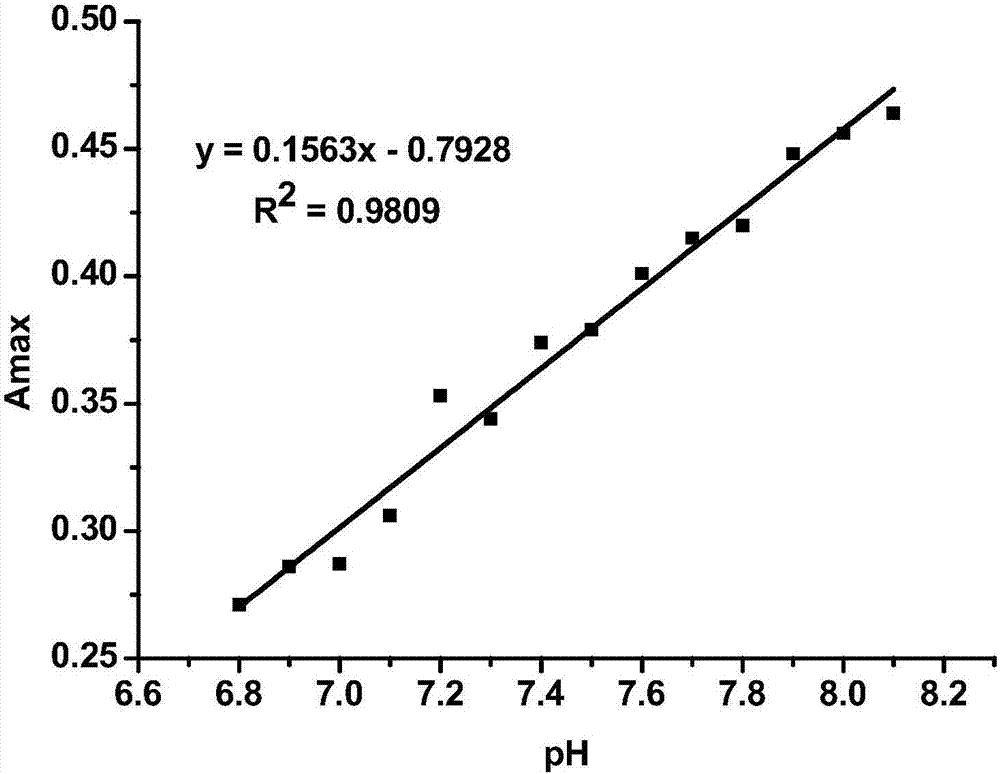
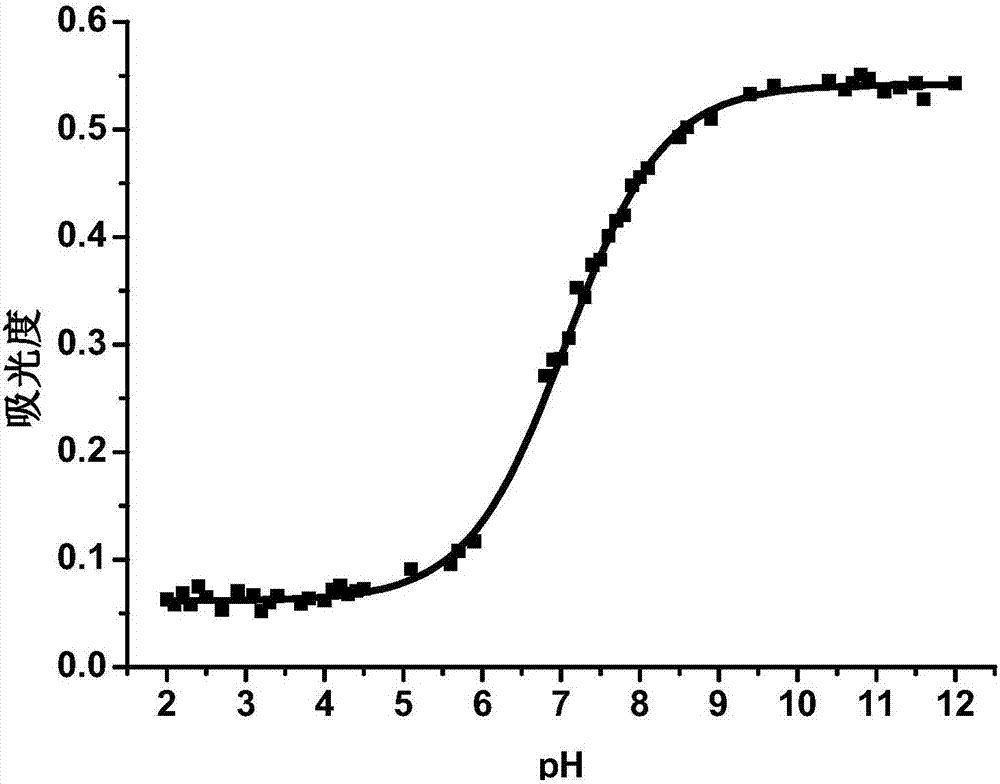
Image
Examples
specific Embodiment approach 1
[0034] Embodiment 1: The structural formula of the phenanthrene imidazole-fluorescein pH fluorescent probe containing two hydroxyl groups in this embodiment is:
[0035]
specific Embodiment approach 2
[0036] Specific embodiment two: the preparation method of the phenanthroimidazole-fluorescein pH fluorescent probe containing two hydroxyl groups described in specific embodiment one is carried out according to the following steps:
[0037] One, the synthesis of intermediate compound I:
[0038] Add phenanthrenequinone, m-nitrobenzaldehyde, aniline, and ammonium acetate into the reactor according to the ratio of 1:(1.0~2.0):(1.0~2.0):(2~5) substance amount, and then add glacial acetic acid as As a solvent, heat up to 80-130°C and stir for 4-12 hours, then cool to room temperature, add water to the reactor, adjust the pH value to 8-10, filter with suction, collect the yellow solid, dry it, and reconstitute it with ethyl acetate. Crystallization, after suction filtration and drying, intermediate compound I 2-(3-nitrophenyl)phenanthroimidazole was obtained;
[0039] Two, the synthesis of intermediate compound II:
[0040] Take intermediate compound I, Raney nickel and mass perc...
specific Embodiment approach 3
[0045] Embodiment 3: This embodiment is different from Embodiment 2 in that the reaction temperature in step 1 is 95-100° C., and the reaction time is 10-12 hours. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com