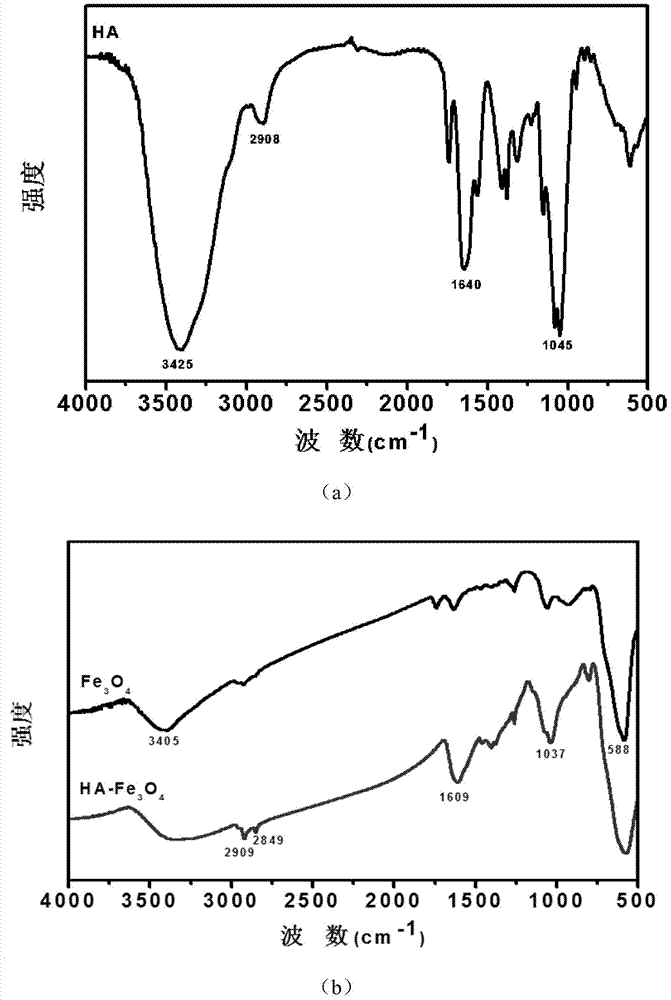
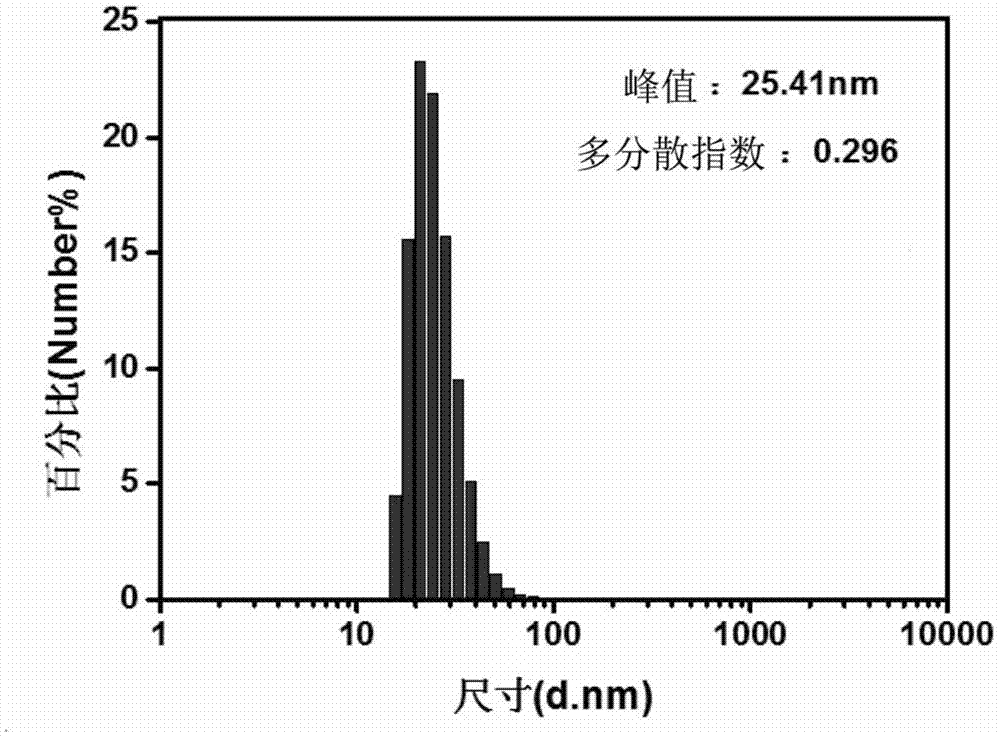
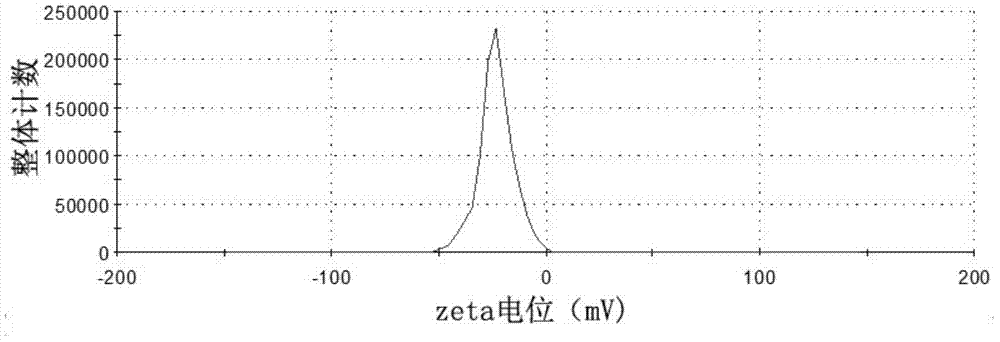
Tumor-targeted T1-T2 double nuclear magnetic resonance imaging contrast agent and preparation method and application thereof
A nuclear magnetic resonance imaging, T1-T2 technology, applied in preparations for in vivo tests, pharmaceutical formulas, emulsion delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] This embodiment adopts the following steps to prepare T 1 -T 2 Dual MRI contrast agents:
[0064] (1) Dissolve 0.5 g of HA in 50 mL of deionized water, place it in a three-necked flask, pass nitrogen gas through the reaction system for 30 minutes, and raise the temperature of the oil bath to 102°C to obtain reaction system A; wherein, the molecular formula of HA used is as follows:
[0065]
[0066] Among them, n=17~290,
[0067] (2) Take 0.1459g of FeCl 3 ·6H 2 O with 0.0715g FeCl 2 4H 2 O was dissolved in 2 mL of 1M hydrochloric acid solution to obtain solution B;
[0068] (3) Inject solution B into reaction system A in step (1), slowly add 15 mL of ammonia water with a mass fraction of 27% dropwise until pH = 10; reflux at high temperature for 40 minutes to obtain reaction system C;
[0069] (4) After the reaction system C in step (3) is cooled to room temperature, all the reaction solution is transferred to a dialysis bag, and after dialysis with deionized...
Embodiment 2
[0083] This embodiment adopts the following steps to prepare T 1 -T 2 Dual MRI contrast agents:
[0084] (1) Dissolve 0.25g of HA in 50mL of deionized water, place it in a three-necked flask, pass nitrogen to the reaction system for 20 minutes, and raise the temperature of the oil bath to 80°C to obtain reaction system A; wherein, the molecular formula of HA used is as follows:
[0085]
[0086] Among them, n=17~290,
[0087] (2) Take 0.1459g of FeCl 3 ·6H 2 O with 0.0804g FeCl 2 4H 2 O was dissolved in 2 mL of 1M hydrochloric acid solution to obtain solution B;
[0088] (3) Inject solution B into reaction system A in step (1), slowly add 15 mL of ammonia water with a mass fraction of 25% dropwise until pH=10; reflux at high temperature for 40 minutes to obtain reaction system C;
[0089] (4) After the reaction system C in step (3) is cooled to room temperature, all the reaction solution is transferred to a dialysis bag, and after dialysis with deionized water at 20°...
Embodiment 3
[0092] This embodiment adopts the following steps to prepare T 1 -T 2 Dual MRI contrast agents:
[0093] (1) Dissolve 2 g of HA in 50 mL of deionized water and place it in a three-necked flask. The reaction system is ventilated with nitrogen for 50 minutes, and the oil bath is heated to 120° C. to obtain reaction system A; wherein, the molecular formula of HA used is as follows:
[0094]
[0095] Among them, n=17~290,
[0096] (2) Take 0.1459g of FeCl 3 ·6H 2 O with 0.0537g FeCl 2 4H 2 O was dissolved in 2 mL of 1M hydrochloric acid solution to obtain solution B;
[0097] (3) Inject solution B into reaction system A in step (1), slowly add 15 mL of ammonia water with a mass fraction of 28% dropwise until pH = 10; reflux at high temperature for 120 minutes to obtain reaction system C;
[0098] (4) After the reaction system C in step (3) is cooled to room temperature, all the reaction solution is transferred to a dialysis bag, and after dialysis with deionized water at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hydrated particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com