Magnetic particle chemiluminescence immunoassay kit of free thyroxine
A technique of free thyroxine and chemiluminescence immunity, which is applied in the field of immunoassay medicine, can solve the problems that cannot be widely used in clinical diagnosis and scientific research work, and is limited in popularization and use, and achieves simple and reliable pretreatment process, convenient use and simple structure. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
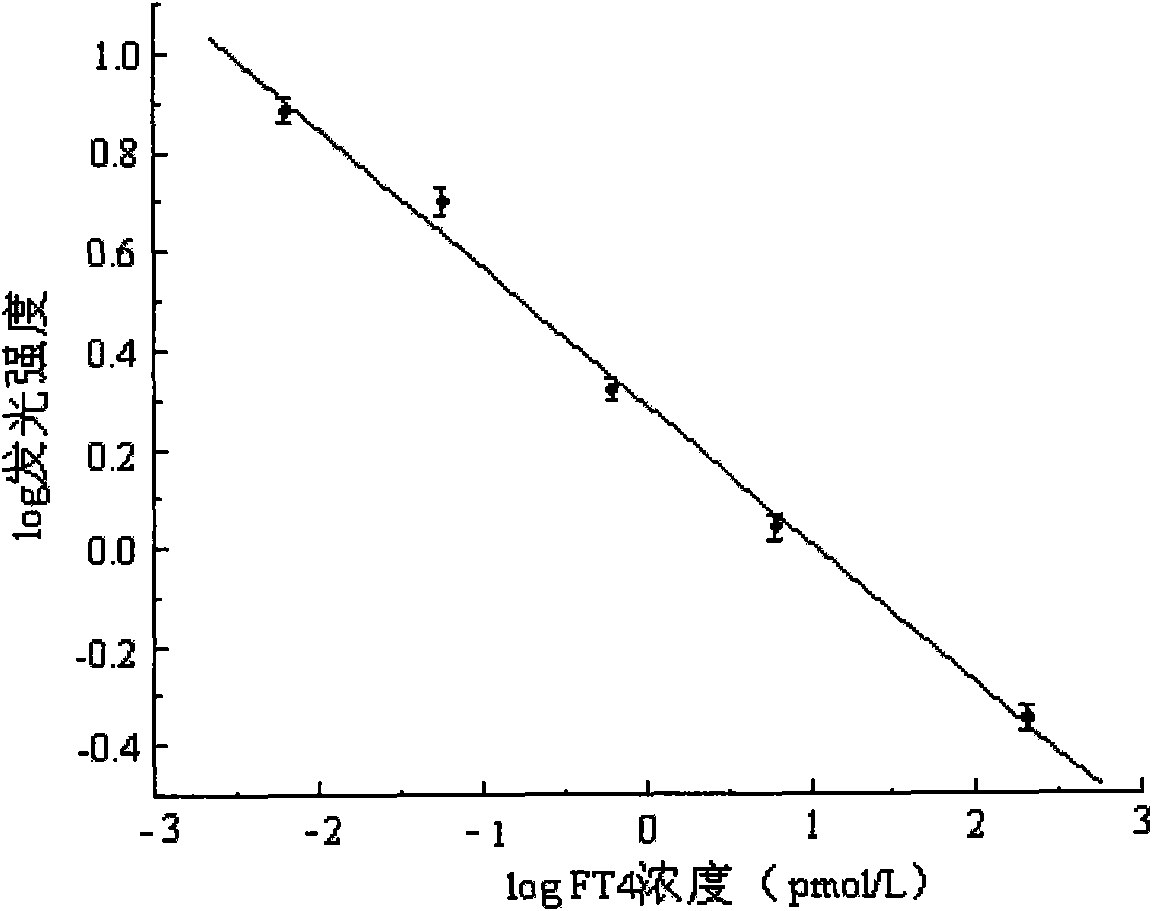
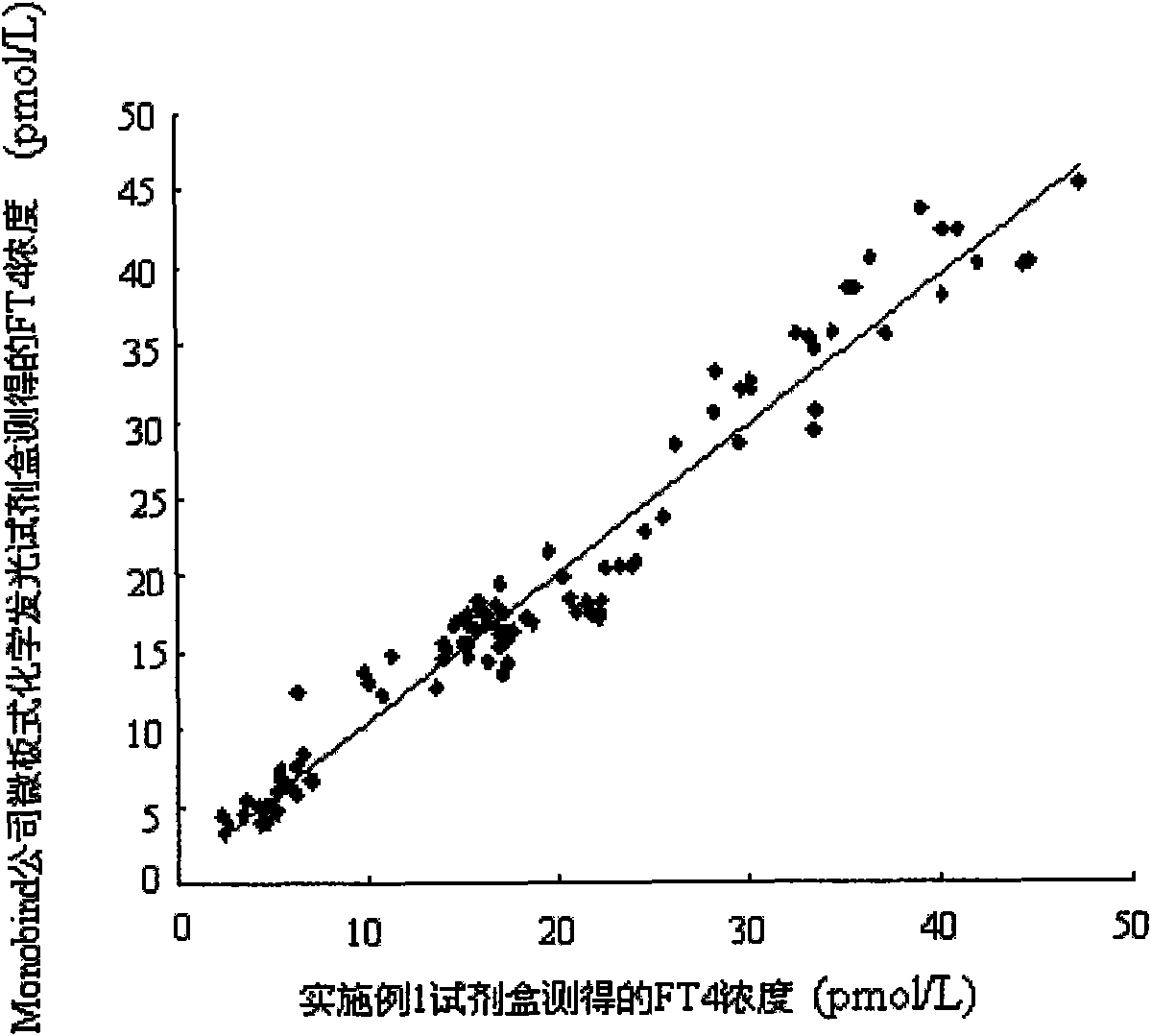
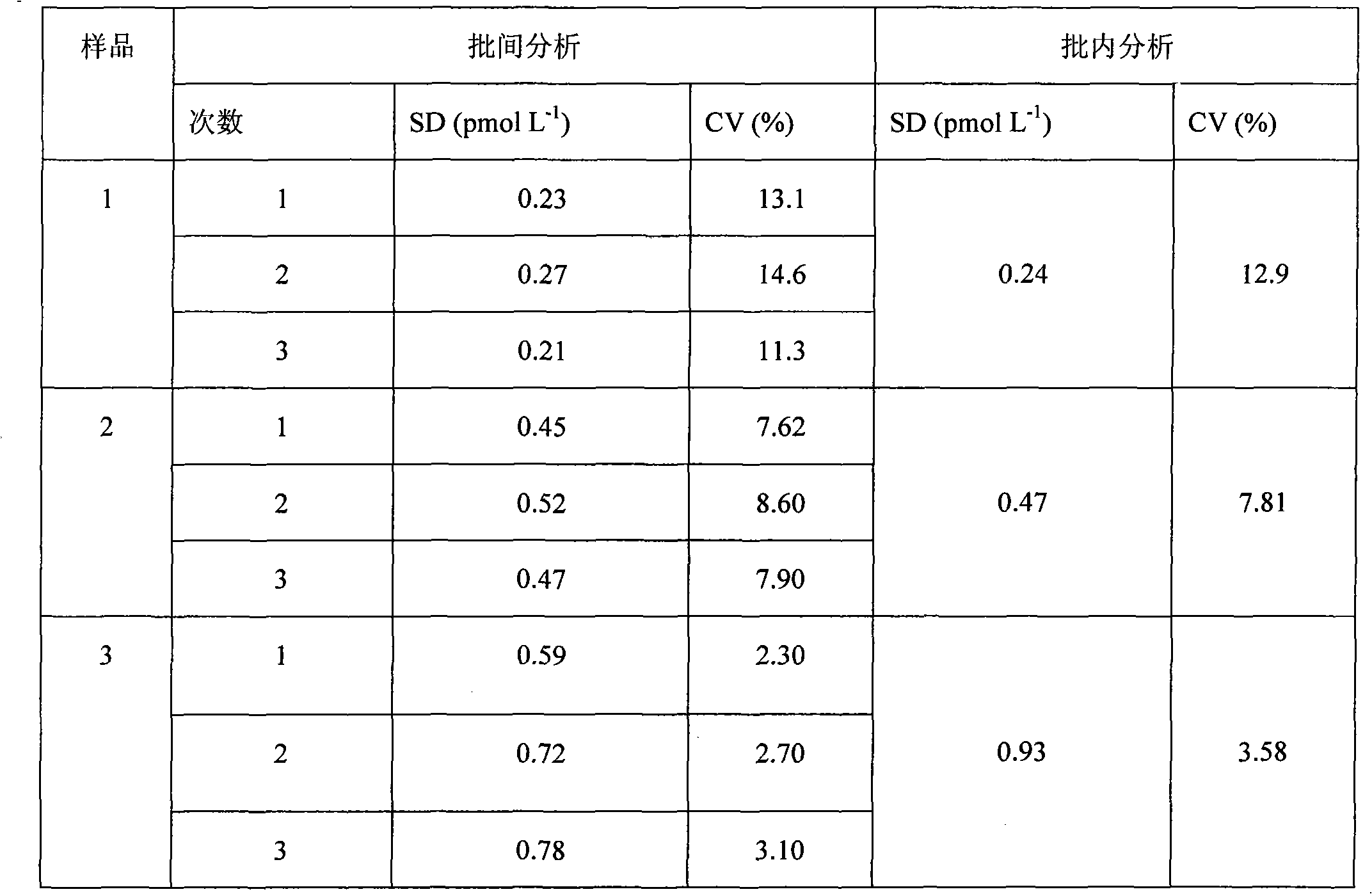
[0058] Embodiment 1 FT of the present invention 4 Preparation of Magnetic Particle Chemiluminescent Immunoassay Assay Kit I
[0059] 1), FT 4 Preparation of calibrator
[0060] A) Preparation of hormone-free human serum: put 60mL of normal human serum in equal parts into four volumetric flasks, then add 8.0g of activated carbon respectively, mix evenly with a vortex mixer by inversion, shake for 8h, centrifuge at 6000rpm for 20min, and The supernatant was filtered, and the preservative Proclin-300 with a volume percentage concentration of 0.5‰ was added to the filtrate, and it was frozen.
[0061] B) Add different amounts of FT to the hormone-free human serum obtained in step A) 4 The pure product was diluted into a calibration product, and after the concentration was determined by the radioimmunoassay method, the concentrations were divided into the following order: 0pmol / L, 1.59pmol / L, 3.45pmol / L, 15.6pmol / L, 37.5pmol / L, and 122pmol / L 6 bottles of FT 4 calibrator.
[0...
Embodiment 2
[0082] Embodiment 2 FT of the present invention 4 Preparation of Magnetic Particle Chemiluminescence Immunoassay Assay Kit II
[0083] 1), FT 4 The preparation of calibrator is the same as embodiment 1
[0084] 2), preparation of anti-FITC monoclonal antibody coated magnetic particle solution
[0085] Activate the magnetic particles with a particle size of 2.0 μm with glutaraldehyde, stir at room temperature, and mix for 2 hours, then strengthen the magnetic field with a strength of 2000 gauss, let it stand for 25 minutes, pour out the supernatant, and use 0.01mol of pH value 7.4 Wash 4 times with / L phosphate buffer solution, and use this solution to suspend at a concentration of 50 mg / mL; add 80 μg of anti-FITC monoclonal antibody to each ml of suspension, stir overnight at 4°C, and then apply a magnetic field with a magnetic field strength of 2000 Gaussian, let it stand for 15min, pour out the supernatant, and use 0.02mol / L phosphate buffer (pH is 7.2) containing mass pe...
Embodiment 3
[0104] Embodiment 3 FT of the present invention 4 Preparation of Magnetic Particle Chemiluminescence Immunoassay Assay Kit III
[0105] 1), FT 4 The preparation of calibrator is the same as embodiment 1
[0106] 2), preparation of anti-FITC monoclonal antibody coated magnetic particle solution
[0107] Activate the magnetic particles with a particle size of 3.0 μm with glutaraldehyde, stir at room temperature, and mix for 2 hours, then strengthen the magnetic field with a strength of 2000 gauss, let it stand for 20 minutes, pour out the supernatant, and use 0.01mol of pH value 7.4 Wash 5 times with / L phosphate buffer solution, and use this solution to suspend at a concentration of 75 mg / mL; add 60 μg of anti-FITC monoclonal antibody to each ml of suspension, stir overnight at 4°C, and then apply a magnetic field with a magnetic field strength of 2000 Gaussian, let it stand for 10min, pour out the supernatant, and use 0.02mol / L phosphate buffer solution (pH is 7.2) containi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com