Novel preparation method of intermediate namely 3-(1-piperidine methyl)phenol of roxatidine acetate hydrochloride
A technology of piperidinylmethyl and phenol, which is applied in the field of intermediate synthesis, can solve the problems of cost increase and raw material m-hydroxybenzaldehyde supply price increase, and achieve the effect of avoiding the increase of production cost and the failure of feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
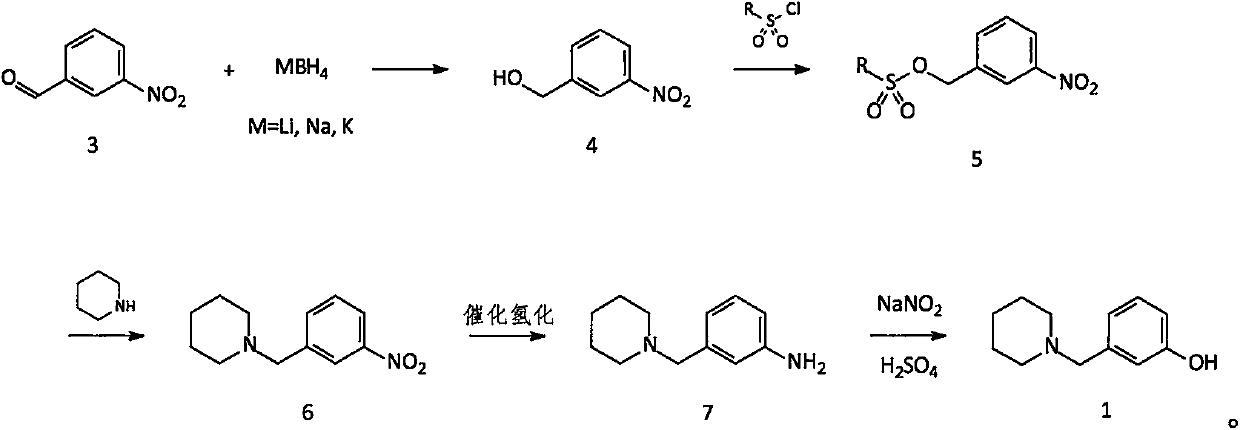
[0043] Embodiment 1: Preparation of 3-(1-piperidylmethyl)nitrobenzene (compound 6)
[0044] Add 1500g of toluene to the reaction flask, then add 604g (4.00mol) of m-nitrobenzaldehyde (compound 3), and then add 6.0g of tetrabutylammonium bromide; cool to about 15°C. Under stirring, an aqueous solution containing 45.5 g (1.20 mol) of sodium borohydride was added dropwise into the reaction liquid, and the temperature was controlled below 30°C. After the dropwise addition was completed, the temperature was controlled at 20-30° C. for 2 hours. Until m-nitrobenzaldehyde is completely reacted.
[0045] After confirming that compound 3 has reacted completely, add 1800g of water to the reaction solution, and then add 820g (4.31mol) of p-toluenesulfonyl chloride under stirring, control the reaction temperature below 30°C, and add dropwise a pre-prepared solution containing 200g (5.00mol) of hydrogen After the aqueous solution of sodium oxide is added dropwise, the temperature is contr...
Embodiment 2
[0049] Embodiment 2: Preparation of 3-(1-piperidylmethyl)aniline (compound 7)
[0050] All the oily matter obtained in Example 1 (calculated on a theoretical basis of 880g, 3.995mol; compound 6) was added to the hydrogenation kettle, then 7700g of methanol was added, and after stirring evenly, 176g of catalyst Raney Ni was added, and then 1100g of methanol was used to dissolve the The catalyst is rinsed clean and added to the hydrogenation kettle. Nitrogen replacement 2-3 times, hydrogen replacement 2-3 times, and then hydrogenation at a pressure of about 3 atm and a temperature of 45-50°C until the reaction is complete.
[0051] After confirming that compound 6 is completely converted into compound 7, cool to room temperature, filter the catalyst, collect the filtrate, concentrate methanol to obtain a solid, and dry to obtain 730 g of compound 7 as an off-white solid (theoretical amount: 760.26 g); yield: 96.0% .
[0052] After the sample was refined and purified with petro...
Embodiment 3
[0057] Embodiment 3: the preparation of 3-(1-piperidinylmethyl)phenol (compound 1)
[0058] Add 1750g of water into the reaction flask, cool to 10-15°C, add 500g (about 5.000mol) of concentrated sulfuric acid dropwise under stirring, and control the temperature below 30°C. After the dropwise addition, cool to 15°C, add 475g (2.50mol) of compound 7 prepared in Example 2, stir to make it completely dissolve, cool down to below 0°C, add dropwise an aqueous solution containing 181g (2.63mol) of sodium nitrite . Control the temperature below 5°C. After the dropwise addition, keep warm at below 5°C and react for 0.5 hr. After TLC confirms that all the compound 7 has reacted, it is ready for use (standby solution 1).
[0059] 1250g of water was added to another reaction flask, and 375g (about 3.75mol) of concentrated sulfuric acid was added dropwise. After the dropwise addition is complete, heat to a temperature of 75°C to 80°C. Add stock solution 1 dropwise through the dropping ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com